Human SerpinI2 Protein (His Tag)
MEPI,PANCPIN,PI14,TSA2004
- 100ug (NPP2494) Please inquiry
| Catalog Number | P11008-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | MEPI,PANCPIN,PI14,TSA2004 |
| Molecular Weight | The recombinant human SERPINI2 consists of 398 amino acids and predictes a molecular mass of 45.5 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhSERPINI2 is approximately 45-55 kDa due to glycosylation. |
| predicted N | Ser 19 |
| SDS-PAGE | 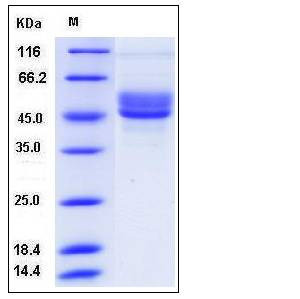 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SERPINI2 (NP_006208.1) (Met 1-Leu 405) was expressed, with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Blood |Fibrinolysis / Thrombolysis |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Serpins are the largest and most diverse family of serine protease inhibitors which are involved in a number of fundamental biological processes such as blood coagulation, complement activation, fibrinolysis, angiogenesis, inflammation and tumor suppression and are expressed in a cell-specific manner. Serpins are a group of proteins with similar structures that were first identified as a set of proteins able to inhibit proteases. The acronym serpin was originally coined because many serpins inhibit chymotrypsin-like serine proteases (serine protease inhibitors). Over 1000 serpins have been identified. Serpin-I2, also known as myoepithelium-derived serine protease inhibitor, Pancreas-specific protein TSA2004, Peptidase inhibitor 14, PI14, SERPINI2 and MEPI, is a secreted protein which belongs to the serpin family. It is expressed in pancreas and adipose tissues. SERPINI2 deficiency directly results in the acinar cell apoptosis and malabsorption. |
| Reference |
