Human TGM3 / Transglutaminase 3 Protein (His Tag)
TGE
- 100ug (NPP4307) Please inquiry
| Catalog Number | P11923-H07B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | TGE |
| Molecular Weight | The recombinant human TGM3 consists of 710 amino acids and predicts a molecular mass of 78.8 kDa. It migrates as an approximately 70 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 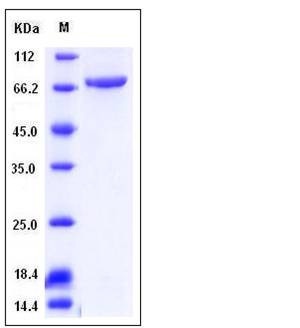 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human TGM3 (Q08188) (Ala 2-Glu 693) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | Measured by its ability to cleave a synthetic peptide Benzyloxycarbonyl-Gln-Gly and NH2OH. The specific activity is > 450 pmoles/min/μg. |
| Research Area | Immunology |Signal Transduction |Metabolism |Amino Acids |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 8.5, 10% gly 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Transglutaminases (TGase) are a family of calcium-dependent acyl-transfer enzymes ubiquitously expressed in mammalian cells and responsible for catalyzing covalent cross-links between proteins or peptides. Transglutaminase 3 (TGM3) is a member of a family of Ca2+-dependent enzymes that catalyze covalent cross-linking reactions between proteins or peptides. TGM3 isoform is widely expressed and is important for epithelial barrier formation. It is a zymogen, requiring proteolysis for activity. Calcium-activated TGM3 can bind, hydrolyze, and is inhibited by GTP, despite lacking structural homology with other GTP binding proteins. TGM3 displays a diffuse cytoplasmic distribution in vitro consistent with its proposed role in the early phase of cornified cell envelope assembly in the cytoplasm. TGM3-driven specific isopeptide bonds between intermediate filaments and KAPs participate to the progressive scaffolding of the hair shaft. Additionally, TGM3 may be a novel prognostic biomarker for esophageal squamous cell carcinoma (ESCC). |
| Reference |
