Human TIMP-1 / TIMP1 Protein
CLGI,EPA,EPO,HCI,TIMP,TIMP-1
- 100ug (NPP4316) Please inquiry
| Catalog Number | P10934-HNAH |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CLGI,EPA,EPO,HCI,TIMP,TIMP-1 |
| Molecular Weight | The recombinant human TIMP1 comprises 184 amino acids with a predicted molecular mass of 21 kDa. It migrates as an approximately 26 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Cys 24 |
| SDS-PAGE | 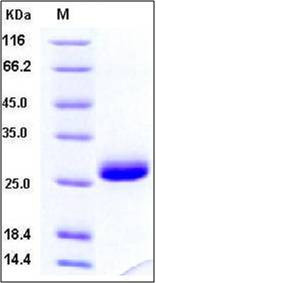 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human TIMP1 (NP_003245.1) (Cys 24-Ala 207) was expressed. |
| Bio-activity | Measured by its ability to inhibit human MMP-2 cleavage of a fluorogenic peptide substrate MCA-PLGL-DPA-AR-NH2(R&D Systems, Catalog # ES001) . The IC50 value is < 6 nM. |
| Research Area | Cancer |Cell cycle |Protease inhibitors|Metalloprotease inhibitors|Tissue Inhibitor of Metalloproteinase (TIMP) |
| Formulation | Lyophilized from sterile 20mM NaAC, 200mM NaCl, pH 5.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | TIMP metallopeptidase inhibitor 1, also known as TIMP-1/TIMP1, Collagenase inhibitor 16C8 fibroblast Erythroid-potentiating activity, TPA-S1TPA-induced proteinTissue inhibitor of metalloproteinases 1, is a natural inhibitors of the matrix metalloproteinases (MMPs), a group of peptidases involved in degradation of the extracellular matrix. TIMP-1/TIMP1 is found in fetal and adult tissues. Highest levels are found in bone, lung, ovary and uterus. Complexes with metalloproteinases and irreversibly inactivates them by binding to their catalytic zinc cofactor. TIMP-1/TIMP1 mediates erythropoiesis in vitro; but, unlike IL-3, it is species-specific, stimulating the growth and differentiation of only human and murine erythroid progenitors. In addition to its inhibitory role against most of the known MMPs, the protein is able to promote cell proliferation in a wide range of cell types, and may also have an anti-apoptotic function. Transcription of this protein encoding gene is highly inducible in response to many cytokines and hormones. In addition, the expression from some but not all inactive X chromosomes suggests that this gene inactivation is polymorphic in human females. This encoding gene is located within intron 6 of the synapsin I gene and is transcribed in the opposite direction. Complexes with metalloproteinases and irreversibly inactivates them by binding to their catalytic zinc cofactor. TIMP-1/TIMP1 is Known to act on MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-11, MMP-12, MMP-13 and MMP-16. |
| Reference |
