Human TIMP2 / TIMP-2 Protein
CSC-21K,DDC8
- 100ug (NPP2539) Please inquiry
| Catalog Number | P10396-HNAH |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CSC-21K,DDC8 |
| Molecular Weight | The recombinant human TIMP2 consists of 195 amino acids and has a predicted molecular mass of 22 kDa. It migrates as an approximately 20 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 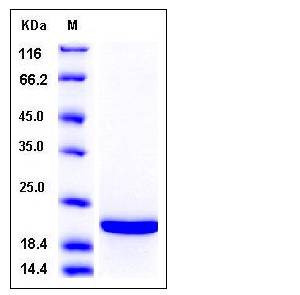 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human TIMP2 (NP_003246.1) (Cys 27-Pro 220) was expressed and purified. |
| Bio-activity | Measured by its ability to inhibit human MMP-2 cleavage of a fluorogenic peptide substrate MCA-PLGL-DPA-AR-NH2(R&D Systems, Catalog # ES001). The IC50 value is < 4 nM. |
| Research Area | Cancer |Cell cycle |Protease inhibitors|Metalloprotease inhibitors|Tissue Inhibitor of Metalloproteinase (TIMP) |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Tissue inhibitors of metalloproteinases (TIMP) family are natural inhibitors of the matrix metalloproteinases (MMPs), the zinc enzymes involved in extracellular matrix maintenance and remodeling. The TIMP family encompasses four members (TIMP1-4), and they inhibit most MMPs by forming non-covalent binary complex. TIMP2 is a 22 kDa non N-glycosylated protein expressed by a variety of cell types, and plays a unique role among TIMP family members owing to its functions to regulate cellular responses to growth factors. Findings establish an unexpected, MMP-independent mechanism for TIMP2 inhibition of endothelial cell proliferation in vitro and reveal an important component of the antiangiogenic effect of TIMP2 in vivo. TIMP-2 thus is critical to the maintenance of tissue homeostasis and is involved in the regulation of tumor microenvironment. |
| Reference |
