Human TPP1 / CLN2 Protein (His Tag)
CLN2,GIG1,LPIC,SCAR7,TPP-1
- 100ug (NPP2552) Please inquiry
| Catalog Number | P13507-H08B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | CLN2,GIG1,LPIC,SCAR7,TPP-1 |
| Molecular Weight | The secreted recombinant human TPP1 (pro form) comprises 554 amino acids and has a predicted molecular mass of 60.7 kDa. The apparent molecular mass of rhTPP1 is approximately 60 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ser 20 |
| SDS-PAGE | 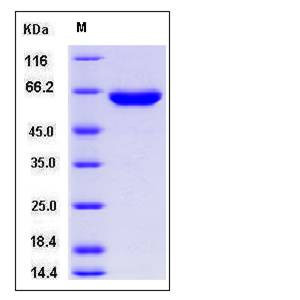 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the pro form of human TPP1 (AAH14863.1) (Met 1-Pro 563) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Neuroscience |Neurology process |Neurodegeneration and Neurodegenerative Disease |Others in Neurodegeneration and Neurodegenerative Disease |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 7.4, 10% gly 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Tripeptidyl-peptidase 1 (TPP1 / CLN2) is a member of the sedolisin family of serine proteases. The protease functions in the lysosome to cleave N-terminal tripeptides from substrates, and has weaker endopeptidase activity. It is synthesized as a catalytically-inactive enzyme which is activated and auto-proteolyzed upon acidification. TPP1 / CLN2 May act as a non-specific lysosomal peptidase which generates tripeptides from the breakdown products produced by lysosomal proteinases. Defects in TPP1 / CLN2 are the cause of neuronal ceroid lipofuscinosis type 2 (CLN2), a form of neuronal ceroid lipofuscinosis which is associated with the failure to degrade specific neuropeptides and a subunit of ATP synthase in the lysosome. Neuronal ceroid lipofuscinoses are progressive neurodegenerative, lysosomal storage diseases characterized by intracellular accumulation of autofluorescent liposomal material, and clinically by seizures, dementia, visual loss, and/or cerebral atrophy. |
| Reference |
