Human TPST1 Protein (His Tag)
TANGO13A
- 100ug (NPP4333) Please inquiry
| Catalog Number | P11258-H07H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | TANGO13A |
| Molecular Weight | The recombinant human TPST1 consists of 361 amino acids and has a calculated molecular mass of 41.7 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhTPST1 is approximately 45-48 kDa due to glycosylation. |
| predicted N | His |
| SDS-PAGE | 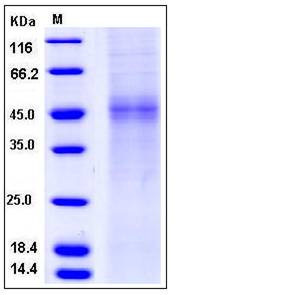 |
| Purity | > 80 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human TPST1 (NP_003587.1) extracellular domain (Gln 26-Glu 370) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Atherosclerosis |Vascular Inflammation |Leukocyte recruitment |Other in Leukocyte recruitment |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Protein-tyrosine sulfotransferase 1, also known as Tyrosylprotein sulfotransferase 1 and TPST1, is a single-pass type I I membrane protein which belongs to the protein sulfotransferase family. Tyrosine O-sulfation is a common posttranslational modification of proteins in all multicellular organisms. This reaction is mediated by a Golgi enzyme activity called tyrosylprotein sulfotransferase (TPST) that catalyzes the transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to tyrosine residues within acidic motifs of polypeptides. Tyrosine O-sulfation has been shown to be important in protein-protein interactions in several systems. Tyrosine sulfation is mediated by one of two Golgi isoenzymes, called tyrosylprotein sulfotransferases (TPST-1 and TPST-2). A relatively small number of proteins are known to undergo tyrosine sulfation, including certain adhesion molecules, G-protein-coupled receptors, coagulation factors, serpins, extracellular matrix proteins, and hormones. TPST1 is a human tyrosylprotein sulfotransferase that uses 3'phosphoadenosine-5'phosphosulfate (PAPS) to transfer the sulfate moiety to proteins predominantly designated for secretion. TPST1 bears N-linked glycosyl residues exclusively at position Asn60 and Asn262. TPST1 and TPST2 have distinct biological roles that may reflect differences in their macromolecular substrate specificity. |
| Reference |
