Human TRAIL R1 / CD261 / TNFRSF10A Protein (His & Fc Tag)
APO2,CD261,DR4,MGC9365,TNFRSF10A,TRAILR-1,TRAILR1
- 100ug (NPP4335) Please inquiry
| Catalog Number | P10408-H03H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | APO2,CD261,DR4,MGC9365,TNFRSF10A,TRAILR-1,TRAILR1 |
| Molecular Weight | The recombinant human TNFRSF10A/Fc is a disulfide-linked homodimer. The reduced monomer consists of 378 amino acids and has a predicted molecular mass of 42 kDa. As a result of glycosylation, the apparent molecular mass of rh TNFRSF10A/Fc monomer migrates with an apparent molecular mass of 47 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ala 109 |
| SDS-PAGE | 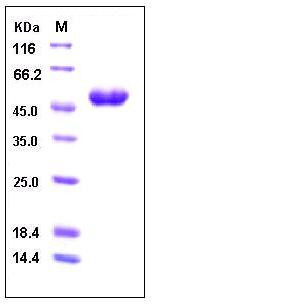 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human TNFRSF10A (NP_003835.2) extracellular domain (Met 1-Asn 239) was fused with the C-terminal polyhistidine-tagged Fc region of human IgG1 at the C-terminus. |
| Bio-activity | 1. Measured by its binding ability in a functional ELISA. Immobilized human TNFSF10 at 10 μg/ml (100 μl/well) can bind human TNFRSF10A Fc Chimera with a linear range of 0.625-20 ng/ml. 2. Measured by its ability to inhibit TRAIL-mediated cytotoxicity using L‑929 mouse fibroblast cells treated with TRAIL. The ED50 for this effect is typically 5-20 ng/ml in the presence of 20 ng/ml Recombinant Human TRAIL/TNFSF10. |
| Research Area | Cardiovascular |Angiogenesis |Growth Factor & Receptor |Tumor Necrosis Factor (TNF) & Receptor |TNF Receptor |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Tumor necrosis factor receptor superfamily, member 10a (TRAIL R1), also known as TRAIL receptor 1 (TRAIL R1) or CD261 antigen, is a member of the TNF-receptor superfamily. This receptor is activated by tumor necrosis factor-related apoptosis inducing ligand (TNFSF10/TRAIL), and thus transduces cell death signal and induces cell apoptosis. Studies with FADD-deficient mice suggested that FADD, a death domain containing adaptor protein, is required for the apoptosis mediated by this protein. TRAIL R1/CD261/TNFRSF10A serves as a receptor for the cytotoxic ligand TNFSF10/TRAIL. The adapter molecule FADD recruits caspase-8 to the activated receptor. The resulting death-inducing signaling complex (DISC) performs caspase-8 proteolytic activation which initiates the subsequent cascade of caspases (aspartate-specific cysteine proteases) mediating apoptosis. TRAIL R1 can promote the activation of NF-kappa-B. TRAIL R1/CD261/TNFRSF10A induces apoptosis of many transformed cell lines but not of normal tissues, even though its death domain-containing receptor, DR4, is expressed on both cell types. |
| Reference |
