Human TREM1 Protein (His Tag)
CD354,TREM-1
- 100ug (NPP4342) Please inquiry
| Catalog Number | P10511-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CD354,TREM-1 |
| Molecular Weight | The secreted recombinant human TREM1 consists of 191 amino acids and has a predicted molecular mass of 21.8 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhTREM1 is approximately 38-42 kDa due to glycosylation. |
| predicted N | Ala 21 |
| SDS-PAGE | 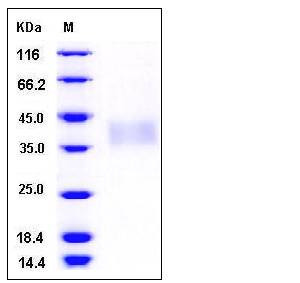 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Arg 200) of human TREM1 (NP_061113.1) was fused with the a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Inflammatory Mediators |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | TREM1 (triggering receptor expressed on myeloid cells) is a type I transmembrane protein with a single Ig-like domain, and is selectively expressed on blood neutrophils and a subset of monocytes. As a member of the growing family of receptors related to NK cell receptors, TREM1 activates downstream signaling events with the help of an adapter protein called DAP12. Expression of TREM1 is up-regulated by bacterial LPS, a ligand for TLR4, as well as lipoteichoic acid. Although its natural ligand has not been identified, engagement of TREM1 with agonist mAbs triggers secretion of the proinflammatory cytokines TNF-α and IL-1β, as well as chemokines such as IL-8 and monocyte chemoattractant protein (MCP)-1. Intracellularly, TREM1 induces Ca2+ mobilization and tyrosine phosphorylation of extracellular signal-related kinase 1 (ERK1), ERK2 and phospholipase C-γ. In an animal model of LPS-induced septic shock, blockade of TREM1 signaling inhibited hyperresponsiveness and death. Thus, it has been demonstrated that TREM1 performs a critical function in immune responses involved in host defense against microbial challenges, and is suggested to be a potential therapeutic target for septic shock. |
| Reference |
