Human TWF1 / PTK9 / Twinfilin-1 Protein
A6,MGC23788,MGC41876,PTK9
- 100ug (NPP4360) Please inquiry
| Catalog Number | P11829-HNCE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | A6,MGC23788,MGC41876,PTK9 |
| Molecular Weight | The recombinant human TWF1 consists of 254 amino acids and has a calculated molecular mass of 29 kDa. It migrates as an approximately 36 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 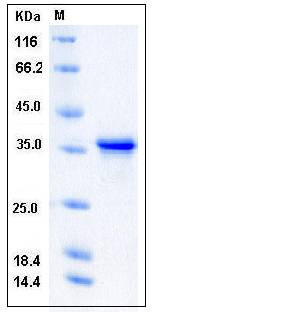 |
| Purity | > 94 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human TWF1 isoform 4 (Q12792-4) (Met 1-Asp 252) was expressed and purified, with additional two amino acids (Gly & Pro) at the N-terminus. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Protein Kinase |Intracellular Kinase |Other Intracellular Protein Kinases |
| Formulation | Lyophilized from sterile PBS, pH 7.4, 10% glycerol 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Twinfilin-1, also known as Protein A6, Protein tyrosine kinase 9, TWF1 and PTK9, is a cytoplasm protein which belongs to the actin-binding proteins ADF family and Twinfilin subfamily. Twinfilin-1 (TWF1 / PTK9 ) is a highly conserved actin monomer-binding protein that regulates cytoskeletal dynamics in organisms from yeast to mammals. In addition to the mammalian twinfilin-1, a second protein with approximately 65% sequence identity to twinfilin-1 exists in mouse and humans. TWF1 / PTK9 is expressed at high levels in the colon, testis, ovary, prostate and lung. It is expressed at lower levels in the brain, bladder and heart. It is not detected in liver. TWF1 / PTK9 is an actin-binding protein involved in motile and morphological processes. It inhibits actin polymerization, likely by sequestering G-actin. By capping the barbed ends of filaments, it also regulates motility. TWF1 / PTK9 seems to play an important role in clathrin-mediated endocytosis and distribution of endocytic organelles. |
| Reference |
