Human Troponin C / TNNC1 Protein
CMD1Z,CMH13,TN-C,TNC,TNNC
- 100ug (NPP2556) Please inquiry
| Catalog Number | P11011-HNAE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CMD1Z,CMH13,TN-C,TNC,TNNC |
| Molecular Weight | The recombinant human TNNC1 consists of 161 amino acids and has a predicted molecular mass of 18.4 kDa. The apparent molecular mass of it is approximately 20 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ser 21 |
| SDS-PAGE | 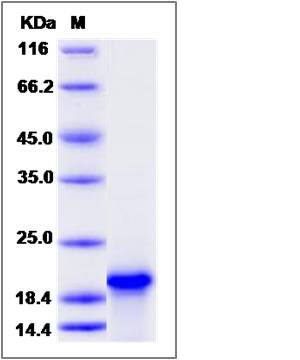 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human TNNC1 (NP_003271.1) (Met1-Glu161) was expressed and purified. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Cytoskeleton / ECM |Cytoskeletal Proteins |Microfilaments |Actin etc | |
| Formulation | Lyophilized from sterile 150 mM NaCl, 10 mM Na2HPO4, pH 7.5. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Troponin I, also known as TNNC1, is part of the troponin complex. This complex contains 3 subunits: troponin I (TnI), troponin T (TnT) and troponin C (TnC). Troponin I is the inhibitory subunit, blocking actin-myosin interactions and thereby mediating striated muscle relaxation. It binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Because of it myosin cannot bind actin in relaxed muscle. When calcium binds to the Troponin C it causes conformational changes which lead to dislocation of troponin I and finally tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. |
| Reference |
