Human Trypsin-3 / PRSS3 Protein (His Tag)
MTG,PRSS4,RP11-176F3.3,T9,TRY3,TRY4
- 100ug (NPP2559) Please inquiry
| Catalog Number | P11866-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | MTG,PRSS4,RP11-176F3.3,T9,TRY3,TRY4 |
| Molecular Weight | The secreted recombinant pro form of the human PRSS3 consists of 243 amino acids and predictes a molecular mass of 26.6 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhPRSS3 is approximately 33 kDa due to glycosylation. |
| predicted N | Val 16 |
| SDS-PAGE | 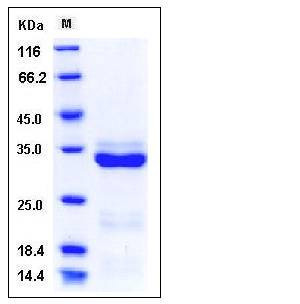 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PRSS3 isoform c (P35030-3) (Met 1-Ser 247) was expressed, with a polyhistidine tag at the C-terminus. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, Mca-RPKPVE-Nval-WRK(Dnp)-NH2 (AnaSpec, Catalog#27114) . The specific activity is >4,000 pmoles/min/μg. (Activation description: The proenzyme needs to be activated by enteropeptidase for an activated form) |
| Research Area | |
| Formulation | Lyophilized from sterile 50mM MES, 0.6M NaCl, pH 5.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Trypsin-3, also known as Trypsin III, brain trypsinogen, Serine protease 3 and PRSS3, is a secreted protein which belongs to the peptidase S1 family. Trypsin-3 / PRSS3 is expressed is in pancreas and brain. It contains one peptidase S1 domain. Trypsin-3 / PRSS3 can degrade intrapancreatic trypsin inhibitors that protect against CP. Genetic variants that cause higher mesotrypsin activity might increase the risk for chronic pancreatitis (CP). A sustained imbalance of pancreatic proteases and their inhibitors seems to be important for the development of CP. The trypsin inhibitor-degrading activity qualified PRSS3 as a candidate for a novel CP susceptibility gene. Trypsin-3 / PRSS3 has been implicated as a putative tumor suppressor gene due to its loss of expression, which is correlated with promoter hypermethylation, in esophageal squamous cell carcinoma and gastric adenocarcinoma. |
| Reference |
