Human USP5 / ISOT Protein (His Tag)
ISOT
- 100ug (NPP4376) Please inquiry
| Catalog Number | P12772-H08B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | ISOT |
| Molecular Weight | The recombinant human USP5 consists of 846 amino acids and predicts a molecular mass of 94.7 kDa. It migrates as an approximately 100 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 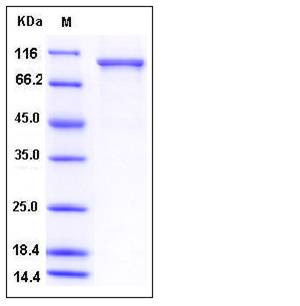 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human USP5 isoform short (P45974-2) (Met 1-Ser 835) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |Histone Modifying Enzymes |Ubiquitylation |Deubiquitination |
| Formulation | Lyophilized from sterile 50mM Tris, 100mM NaCl, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ubiquitin carboxyl-terminal hydrolase 5, also known as Deubiquitinating enzyme 5, Isopeptidase T, Ubiquitin thiolesterase 5, Ubiquitin-specific-processing protease 5, ISOT and USP5, is a member of the peptidase C19 family. USP5 contains 2 UBA domains and one UBP-type zinc finger. The UBP-type zinc finger domain interacts selectively with an unmodified C-terminus of the proximal ubiquitin. Both UBA domains are involved in polyubiquitin recognition. The UBP-type zinc finger domain crystallizes as a dimer linked by a disulfide bond between the Cys-195 residues of both molecules, but there is no evidence that the full-length USP5 exists as a dimer. USP5 cleaves linear and branched multiubiquitin polymers with a marked preference for branched polymers. USP5 is involved in unanchored 'Lys-48'-linked polyubiquitin disassembly. It binds linear and 'Lys-63'-linked polyubiquitin with a lower affinity. Knock-down of USP5 causes the accumulation of p53/TP53 and an increase in p53/TP53 transcriptional activity because the unanchored polyubiquitin that accumulates is able to compete with ubiquitinated p53/TP53 but not with MDM2 for proteasomal recognition. |
| Reference |
