Human USP7 / HAUSP Protein (aa 208-560)
HAUSP,TEF1
- 100ug (NPP4378) Please inquiry
| Catalog Number | P11681-HNCB |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | HAUSP,TEF1 |
| Molecular Weight | The recombinant human USP7 (208-560) consists of 355 amino acids and has a calculated molecular mass of 41 kDa as estimated in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 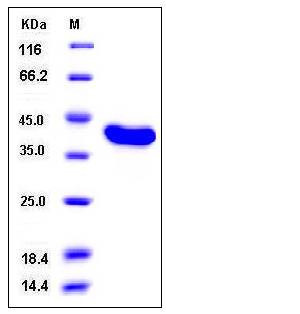 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the amino acid sequence (Lys 208-Glu 560) of human USP7 (NP_003461.2) expressed and purified, with two additional aa (Gly & Pro) at the N-terminus. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Metabolism |Pathways and Processes |Metabolism processes |Apoptosis | |
| Formulation | Lyophilized from sterile 50mM Tris, 100mM NaCl, 0.5mM PMSF, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ubiquitin carboxyl-terminal hydrolase 7, also known as Ubiquitin thioesterase 7, Herpesvirus-associated ubiquitin-specific protease, Ubiquitin-specific-processing protease 7, USP7 and HAUSP, is a widely expressed protein which belongs to the peptidase C19 family. USP7 is a member of the family of deubiquitinating enzymes. It is involved in the regulation of stress response pathways, epigenetic silencing and the progress of infections by DNA viruses. USP7 is a protein with a cysteine peptidase core, N- and C-terminal domains required for protein-protein interactions. USP7 contributes to epigenetic silencing of homeotic genes by Polycomb (Pc). USP7 cleaves ubiquitin fusion protein substrates. It deubiquitinates TP53/p53 and MDM2 and strongly stabilizes TP53 even in the presence of excess MDM2. USP7 also induces TP53-dependent cell growth repression and apoptosis. USP7 has key roles in the p53 pathway whereby it stabilizes both p53 and MDM2. Herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 stimulates lytic infection and the reactivation of quiescent viral genomes. ICP0 interacts very strongly with USP7. USP7-mediated stabilization of ICP0 is dominant over ICP0-induced degradation of USP7 during productive HSV-1 infection. The biological significance of the ICP0-USP7 interaction may be most pronounced in natural infection situations, in which limited amounts of ICP0 are expressed. |
| Reference |
