Human Urokinase / PLAU Protein (His Tag)
ATF,BDPLT5,QPD,u-PA,UPA,URK
- 100ug (NPP4373) Please inquiry
| Catalog Number | P10815-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ATF,BDPLT5,QPD,u-PA,UPA,URK |
| Molecular Weight | The secreted recombinant human PLAU comprises 422 amino acids with a predicted molecular mass of 46 kDa. As a result of glycosylation, rhPLAU migrates as three bands corresponding to the long A chain, B chain and unprocessed full-length PLAU with the molecular mass of 18, 32 and 50 kDa respectively in SDS-PAGE under reducing conditions. |
| predicted N | Ser 21 |
| SDS-PAGE | 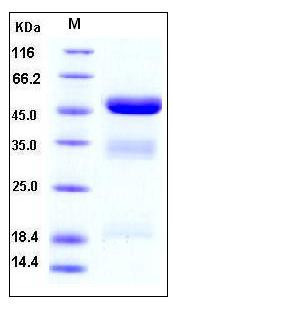 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PLAU (NP_002649.1) (Met 1-Leu 431) with a C-terminal polyhistidine tag was expressed. |
| Bio-activity | Measured by its binding ability in a functional ELISA . Immobilized human uPA at 5 μg/ml (100 μl/well) can bind mouse PLAUR with a linear ranger of 1.6-40 ng/ml. |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Coagulation |Coagulation Cascade |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Plasminogen activator, urokinase, also known as PLAU and uPA, is a serine protease which converts plasminogen to plasmin, a broad-spectrum protease active on extracellular matrix (ECM) components. It is involved in complement activation, cell migration, wound healing, and generation of localized extracellular proteolysis during tissue remodelling, pro-hormone conversion, carcinogenesis and neoplasia. Like many components of the blood coagulation, fibrinolytic and complement cascades, uPA has a modular structure, including three conserved domains: a growth factor-like domain (GFD, residues 1-49), a kringle domain (residues 50-131), linked by an interdomain linker or "connecting peptide" (CP, residues 132-158) to the serine protease domain (residues 159-411). uPA and its receptor (uPAR) have been implicated in a broad spectrum of pathophysiological processes, including fibrinolysis, proteolysis, inflammation, atherogenesis and plaque destabilization, all of which are involved in the pathogenesis of MI (myocardial infarction). The role of uPA is not only linked to its action as an enzyme. In fact, the mere binding of uPA on the cell surface also brings about two events that broaden the spectrum of its biological functions: (1) a conformational change of the receptor, which, in turn, affects its interaction with other proteins; (2) a signal transduction which modulates the expression of apoptosis-related genes. Besides its applications as a thrombolytic agent and as a prognostic marker for tumors, uPA may provide the basis for other therapies, as the structure of the receptor-binding domain of uPA has become a model for the design of anti-cancer molecules. Because of the causal involvment of uPA in cancer invasion and metastasis, the blockade of uPA interactions and activity with specific inhibitors is of interest for novel strategies in cancer therapy. |
| Reference |
