Human VE-Cadherin / CD144 / CDH5 Protein (His & Fc Tag)
7B4,CD144
- 100ug (NPP4385) Please inquiry
| Catalog Number | P10433-H03H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | 7B4,CD144 |
| Molecular Weight | The recombinant human CDH5/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 815 amino acids after removal of the signal peptide and has a predicted molecular mass of 92 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rh CDH5/Fc monomer is approximately 120 kDa due to glycosylation. |
| predicted N | Ala 26 |
| SDS-PAGE | 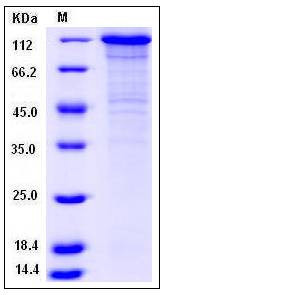 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain of human CDH5 (NP_001786.2) (Met 1-Gln 593) was fused with the C-terminal polyhistidine-tagged Fc region of human IgG1 at the C-terminus. |
| Bio-activity | Measured by the ability of the immobilized protein to support the adhesion of MCF-7 human breast adenocarcinoma cells. When 5 x 10E4 cells/well are added to Recombinant Human Cadherin-5 coated plates (0.8 μg/mL with 100 μL/well), approximately >50% will adhere after 1 hour at 37℃. |
| Research Area | Cancer |Signal transduction |Cytoskeleton / ECM |Cell Adhesion |Cell Adhesion Molecules |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cadherins (Calcium dependent adhesion molecules) are a class of transmembrane proteins. Cadherin-5, also known as VE-cadherin, CDH5 and CD144, an endothelial specific cell-cell adhesion molecule, plays a pivotal role in the formation, maturation and remodeling of the vascular wall. VE-Cadherin is widely considered to be specific for vascular endothelia in which it is either the sole or the predominant cadherin, often co-existing with N-cadherin. This specificity of VE-cadherin for vascular endothelial cells is important not only in blood and lymph vessel biology and medicine, but also for cell-type-based diagnoses, notably those of metastatic tumors. As a classical cadherin, VE-Cadherin links endothelial cells together by homophilic interactions mediated by its extracellular part and associates intracellularly with the actin cytoskeleton via catenins. Mechanisms that regulate VE-cadherin-mediated adhesion are important for the control of vascular permeability and leukocyte extravasation. In addition to its adhesive functions, VE-Cadherin regulates various cellular processes such as cell proliferation and apoptosis and modulates vascular endothelial growth factor receptor functions. Consequently, VE-cadherin is essential during embryonic angiogenesis. |
| Reference |
