Human VEGF-C Protein (His Tag)
Flt4-L,LMPH1D,VEGF-C,VRP
- 100ug (NPP1601) Please inquiry
| Catalog Number | P10542-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | Flt4-L,LMPH1D,VEGF-C,VRP |
| Molecular Weight | The recombinant mature form of human VEGFC consists of 136 amino acids and has a predicted molecular mass of 15.5 kDa. In SDS-PAGE under reducing conditions, it migrates with an apparent molecular mass of 22-24 kDa due to glycosylation. |
| predicted N | Thr 103 |
| SDS-PAGE | 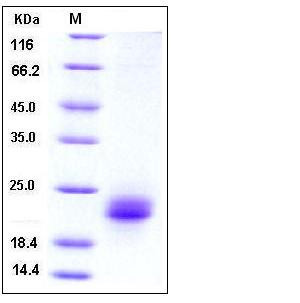 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human VEGFC (NP_005420.1) corresponding to amino acid (Thr 103-Arg 227) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | 1. Measured by its binding ability in a functional ELISA . Scatchard analysis showed the affinity constant (Kd) of recombinant human VEGF-C bound to recombinant human VEGFR3 was 1.4 nM . 2. Measured in a cell proliferation assay using human umbilical vein endothelial cells (HUVEC). The ED50 for this effect is 0.1-0.5μg/mL. |
| Research Area | Cancer |Signal transduction |Growth Factor & Receptor |Vascular Endothelial Growth Factor (VEGF) & Receptor |Vascular Endothelial Growth Factor (VEGF) |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Vascular endothelial growth factor C (VEGF-C) is a member of the VEGF family. Upon biosynthesis, VEGF-C protein is secreted as a non-covalent momodimer in an anti-parellel fashion. VEGF-C protein is a dimeric glycoprotein, as a ligand for two receptors, VEGFR-3 (Flt4), and VEGFR-2. VEGF-C may function in angiogenesis of the venous and lymphatic vascular systems during embryogenesis. VEGF-C protein is over-expressed in various human cancers including breast cancer and prostate cancer. VEGF-C/VEGFR-3 axis, through different signaling pathways, plays a critical role in cancer progression by regulating different cellular functions, such as invasion, proliferation, and resistance to chemotherapy. Thus, targeting the VEGF-C/VEGFR-3 axis may be therapeutically significant for certain types of tumors. |
| Reference |
