Human respiratory syncytial virus (RSV) (A, rsb1734) glycoprotein G / RSV-G Protein (95% Homology) (His Tag)
G
- 100ug (NPP2970) Please inquiry
| Catalog Number | P11070-V08H |
|---|---|
| Organism Species | RSV |
| Host | Human Cells |
| Synonyms | G |
| Molecular Weight | The secreted recombinant human RSV-G comprises 242 amino acids with a predicted molecular mass of 26.7 kDa. As a result of highly glycosylation, the RSV-G protein migrates as an approximately 80-90 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Asn 66 |
| SDS-PAGE | 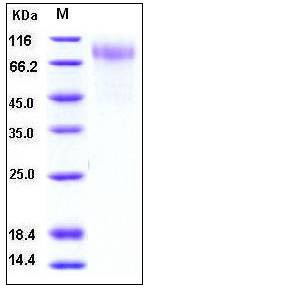 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the glycoprotein G extracellular domain (Asn 66-Arg 297) of human respiratory syncytial virus A (95 % homologous with strain rsb1734) (P27022-1) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Microbiology |Pathogenic microorganism |viruses |animal virus |viral illness |Viral tract respiratory illness | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Human respiratory syncytial virus (HRSV) is the most common etiological agent of acute lower respiratory tract disease in infants and can cause repeated infections throughout life. It is classified within the genus pneumovirus of the family paramyxoviridae. Like other members of the family, HRSV has two major surface glycoproteins (G and F) that play important roles in the initial stages of the infectious cycle. HRSV G protein is a type II glycoprotein of 289-299 amino acids (depending on the virus strain) with a signal/anchor hydrophobic domain and is extensively modified by the addition of both N-and O-linked oligosaccharides to achieve the mature form of 80-90 kDa. The C-terminal ectodomain of the G protein has a central region and four cysteines which are conserved in all HRSV isolates and have been proposed as the putative receptor binding site. The G protein mediates attachment of the virus to the host cell membrane by interacting with heparan sulfate, initiating the infection. As similar to mucins in amino acid compositions, the RSV G protein can interact with host CX3CR1, the receptor for the CX3C chemokine fractalkine, and thus modulates the immune response and facilitate infection. Secreted glycoprotein G helps RSV escape antibody-dependent restriction of replication by acting as an antigen decoy and by modulating the activity of leukocytes bearing Fcgamma receptors. Unlike the other paramyxovirus attachment proteins, HRSV-G lacks both neuraminidase and hemagglutinating activities. |
| Reference |
