Human respiratory syncytial virus (RSV) Fusion protein / RSV-F (Strain RSS-2) Protein (His Tag)
F,HRSVgp08
- 100ug (NPP2962) Please inquiry
| Catalog Number | P40037-V08B |
|---|---|
| Organism Species | RSV |
| Host | Baculovirus-Insect Cells |
| Synonyms | F,HRSVgp08 |
| Molecular Weight | The secreted recombinant human RSV (strain RSS-2) comprises 519 amino acids with a predicted molecular mass of 57.8 kDa. The RSV F0 precursor protein is cleaved into the disulfide-linked F1 and F2 subunits. As a result of glycosylation, the apparent molecular mass of the protein is approximately 63 kDa and 44-53 KDa in SDS-PAGE under reducing conditions, corresponding to the two subunits respectively. |
| predicted N | Phe 22 |
| SDS-PAGE | 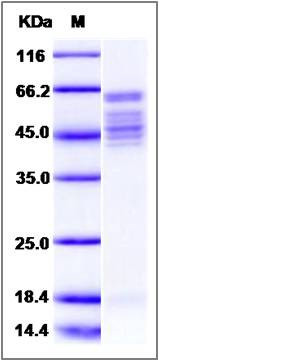 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain of human RSV (strain RSS-2) fusion protein (P11209) (Met 1-Thr 529) was expressed with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Microbiology |Pathogenic microorganism |viruses |animal virus |Virus antigen |RSV viral antigen | |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 7.4, 10% gly 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Human respiratory syncytial virus (HRSV) is the most common etiological agent of acute lower respiratory tract disease in infants and can cause repeated infections throughout life. It is classified within the genus pneumovirus of the family paramyxoviridae. Like other members of the family, HRSV has two major surface glycoproteins (G and F) that play important roles in the initial stages of the infectious cycle. The G protein mediates attachment of the virus to cell surface receptors, while the F protein promotes fusion of the viral and cellular membranes, allowing entry of the virus ribonucleoprotein into the cell cytoplasm. The fusion (F) protein of RSV is synthesized as a nonfusogenic precursor protein (F0), which during its migration to the cell surface is activated by cleavage into the disulfide-linked F1 and F2 subunits. This fusion is pH independent and occurs directly at the outer cell membrane, and the F2 subunit was identifed as the major determinant of RSV host cell specificity. The trimer of F1-F2 interacts with glycoprotein G at the virion surface. Upon binding of G to heparan sulfate, the hydrophobic fusion peptide is unmasked and induces the fusion between host cell and virion membranes. Notably, RSV fusion protein is unique in that it is able to interact directly with heparan sulfate and therefore is sufficient for virus infection. Furthermore, the fusion protein is also able to trigger p53-dependent apoptosis. |
| Reference |
