Human tPA / PLAT Protein
T-PA,TPA
- 100ug (NPP4331) Please inquiry
| Catalog Number | P10157-HNCH2 |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | T-PA,TPA |
| Molecular Weight | The recombinant human tPA β chain consists of 252 amino acids and has a predicted molecular mass of 28 kDa. As a result of glycosylation, the apparent molecular mass of rh tPAβ is approximately 38 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ile 311 |
| SDS-PAGE | 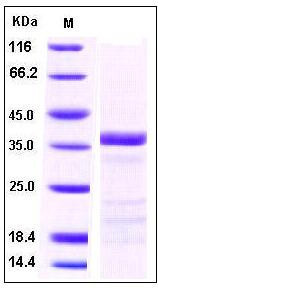 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | The β chain (Ile 311-Pro 562) of mature human tPA (NP_000921.1) was obtained after cleavage of the N-terminal human IgG1 Fc region from the purified chimera. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Fibrinolysis System |
| Formulation | Lyophilized from sterile 100mM Glycine, 10mM NaCl, 50mM Tris, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Tissue plasminogen activator (abbreviated tPA or PLAT), is traditionally viewed as a simple serine protease whose main function is to convert plasminogen into biologically active plasmin. As a protease, tPA plays a crucial role in regulating blood fibrinolysis, in maintaining the homeostasis of extracellular matrix and in modulating the post-translational activation of growth factors. tPA is synthesized and secreted as a single chain polypeptide precursor which is cleaved in turn by plasmin. Proteolytic cleavage at the C-terminal side of Arg275 generates the enzyme composed of two subunits, designated as α and β chains which are held together by a single disulfide bond. Unlike the other members of the chymotrypsin family, tPA has one particular distinction in that the catalytic efficiency of the single-chain enzyme is only slightly lower than that of the proteolytically cleaved form and is therefore not a true zymogen. tPA is found not only in the blood, where its primary function is as a thrombolytic enzyme, but also in the central nervous system (CNS). It participats in a number of physiological and pathological events in the CNS, as well as the role of neuroserpin as the natural regulator of tPA's activity in these processes. Increased or decreased activity of tPA leads to hyperfibrinolysis or hypofibrinolysis, respectively. In addition, as a cytokine, tPA plays a pivotal role in the pathogenesis of renal interstitial fibrosis through diverse mechanisms. Thus, as a fibrogenic cytokine, it promotes the progression of kidney diseases. |
| Reference |
