Human vWF Protein (His Tag)
F8VWF,Von Willebrand Factor,VWD
- 100ug (NPP1619) Please inquiry
| Catalog Number | P10973-H08C |
|---|---|
| Organism Species | Human |
| Host | CHO Stable Cells |
| Synonyms | F8VWF,Von Willebrand Factor,VWD |
| Molecular Weight | The secreted recombinant human vWF consists of 2802 amino acids after removal of the signal peptide and has a predicted molecular mass of 308 kDa. Purified rhvWf exists as both the pro form with the pro peptide (307 kDa) and the mature form (226 kDa), which migrates as doublets with apparent molecular mass of 260 and 350 kDa respectively in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Ala 23 |
| SDS-PAGE | 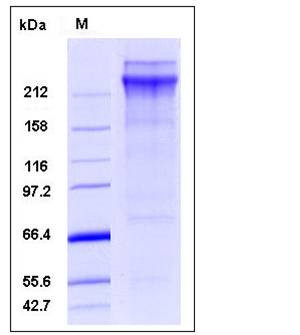 |
| Purity | > 75 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the pro form of human von Willebrand factor (NP_000543.2) (Met 1-Lys 2813) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Immunology |Innate Immunity |Coagulation |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Von Willebrand Factor (VWF) is a multimeric glycoprotein involved in hemostasis in blood, binds receptors on the surface of platelets and in connective tissue, thereby mediating the adhesion of platelets to sites of vascular injury. From studies it appears that VWF protein uncoils under these circumstances, decelerating passing platelets. VWF protein is deficient or defective in von Willebrand disease (VWD) and is involved in a large number of other diseases, including thrombosis, thrombotic thrombocytopenic purpura, Stroke, Heyde's syndrome, possibly hemolytic-uremic syndrome and so on. |
| Reference |
