Mouse AARS / alanyl-tRNA synthetase Protein (His Tag)
AI316495,C76919,sti
- 100ug (NPP3169) Please inquiry
| Catalog Number | P50384-M08B |
|---|---|
| Organism Species | Mouse |
| Host | Baculovirus-Insect Cells |
| Synonyms | AI316495,C76919,sti |
| Molecular Weight | The secreted recombinant mouse AARS consists of 978 amino acids and has a calculated molecular mass of 108.3 kDa. The recombinant protein migrates as an approximately 105 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 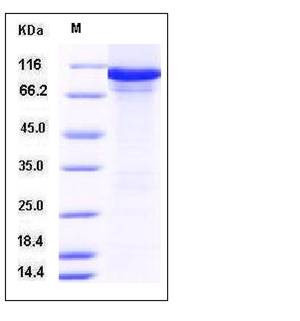 |
| Purity | > 88 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse AARS (Q8BGQ7) (Met 1-Asn 968) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 7.4, 10% glycerol 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Alanyl-tRNA synthetase (AARS) belongs to the family of ligases, specifically those forming carbon-oxygen bonds in aminoacyl-tRNA and related compounds. This enzyme participates in alanine and aspartate metabolism and aminoacyl-tRNA biosynthesis. Alanyl-tRNA synthetase (AlaRS) catalyzes synthesis of Ala-tRNA (Ala) and hydrolysis of mis-acylated Ser- and Gly-tRNA (Ala) at 2 different catalytic sites. Their role is not confined to catalyze the attachment of amino acids to transfer RNAs and thereby establish the rules of genetic code by virtue of matching the nucleotide triplet of anticodon with cognate amino acid. Under apoptotic conditions in cell culture, the full-length enzyme is secreted, and the two cytokine activities can be generated by leukocyte elastase, an extracellular protease. Secretion of this tRNA synthetase may contribute to apoptosis both by arresting translation and producing needed cytokines. This protein could be an attractive target of drugs against bacterial, fungal and parasitic infections. |
| Reference |
