Mouse ACY1 / Aminoacylase-1 Protein (His Tag)
1110014J22Rik,Acy-1
- 100ug (NPP2656) Please inquiry
| Catalog Number | P50008-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | 1110014J22Rik,Acy-1 |
| Molecular Weight | The recombinant mouse ACY1 consists of 402 amino acids and has a calculated molecular mass of 45 kDa as estimated by SDS-PAGE under reducing conditions. |
| predicted N | Gln 18 |
| SDS-PAGE | 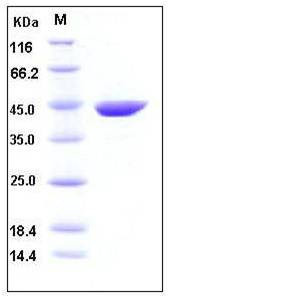 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse ACY1 (Q99JW2) (Met 1-Ser 408) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to cleave N-acetyl-L-Methione (Ac-Met) . The specific activity is >4,000 pmoles/min/μg. |
| Research Area | Developmental Biology |Metabolism |Amino Acids |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Aminoacylase 1 (ACY1), a metalloenzyme that removes amide-linked ACY1 groups from amino acids and may play a role in regulating responses to oxidative stress. Both the C-terminal fragment found in the two-hybrid screen and full-length ACY1 co-immunoprecipitate with SphK1. Though both C-terminal and full-length proteins slightly reduce SphK1 activity measured in vitro, the C-terminal fragment inhibits while full-length ACY1 potentiates the effects of SphK1 on proliferation and apoptosis. It suggested that ACY1 physically interacts with SphK1 and may influence its physiological functions. As a homodimeric zinc-binding enzyme, Aminoacylase 1 catalyzes the hydrolysis of N alpha-acylated amino acids. Deficiency of Aminoacylase 1 due to mutations in the Aminoacylase 1 (ACY1) gene follows an autosomal-recessive trait of inheritance and is characterized by accumulation of N-acetyl amino acids in the urine. |
| Reference |
