Mouse ADAM9 Protein (His Tag)
AU020942,MDC9,mKIAA0021,Mltng
- 100ug (NPP2652) Please inquiry
| Catalog Number | P50044-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | AU020942,MDC9,mKIAA0021,Mltng |
| Molecular Weight | The secreted recombinant mouse ADAM9 consists of 679 amino acids and has a calculated molecular mass of 74.9 kDa. |
| predicted N | Gly 30 |
| SDS-PAGE | 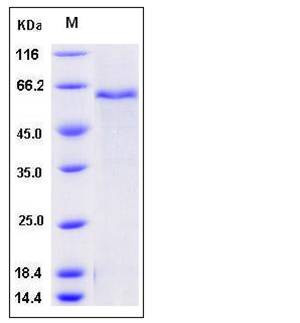 |
| Purity | > 87 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse ADAM9 (Q61072) extracellular domain (Met 1-Asp 697) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Cancer |Invasion microenvironment |Adhesion molecule |Extracelluar matrix |Extracellualr matrix proteases & regulators |A disintegrin and metalloproteinase (ADAM) & ADAMTS | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ADAM9 (A disintegrin and metallopeptidase domain 9, MDC9, meltrin gamma), is a type 1 transmembrane protein that has been associated with cancer development and metastases. ADAM9 is consistently overexpressed in various human cancers, and plays a role in tumorigenesis in mouse models. ADAM9 cleaves and releases a number of molecules with important roles in tumorigenesis and angiogenesis, such as EGF, FGFR2iiib, Tie-2, Flk-1, EphB4, CD40, VCAM-1, and VE-cadherin, and could represent a potential therapeutic target in tumors where it is highly expressed. ADAM9 belongs to a family of transmembrane, disintegrin-containing metalloproteinases involved in protein ectodomain shedding and cell-cell and cell-matrix interactions. ADAM-9 adhesive domain plays a role in regulating the motility of cells by interaction with beta1 integrins and modulates MMP synthesis. |
| Reference |
