Mouse BCL2L1 / Bcl-XL Protein (aa 1-212, His Tag)
Bcl(X)L,bcl-x,Bcl-XL,bcl2-L-1,Bcl2l,BclX
- 100ug (NPP3188) Please inquiry
| Catalog Number | P50012-M08E |
|---|---|
| Organism Species | Mouse |
| Host | E. coli |
| Synonyms | Bcl(X)L,bcl-x,Bcl-XL,bcl2-L-1,Bcl2l,BclX |
| Molecular Weight | The recombinant mouse BCL2L1 comprises 222 amino acids and has a calculated molecular mass of 25.2 kDa. It migrates as an approximately 33 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 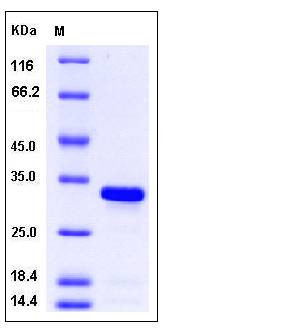 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse BCL2L1 (Q64373-1) (Met 1-Arg 212) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | 1. Measured by its binding ability in a functional ELISA. 2. Immobilized human BID at 10 μg/mL (100 μl/well) can bind biotinylated mouse BCL2L1, The EC50 of biotinylated mouse BCL2L1 is 5.6 ng/mL. 3. Immobilized mouse BID at 10 μg/mL (100 μl/well) can bind biotinylated mouse BCL2L1, The EC50 of biotinylated mouse BCL2L1 is 7.1 ng/mL. |
| Research Area | Cancer |Signal transduction |Metabolism |Pathways and Processes |Metabolism processes |Apoptosis | |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | B-cell lymphoma-extra large (Bcl-xl) is a transmembrane molecule in the mitochondria. Bcl-xL (BCL2L1) , belongs to the Bcl-2 family. Members of the bcl-2 family encode proteins that function either to promote or to inhibit apoptosis. Antiapoptotic members such as Bcl-2 and Bcl-xL prevent PCD in response to a wide variety of stimuli to take part in cancer survival. Conversely, proapoptotic proteins, exemplified by Bax and Bak, can accelerate death and in some instances are sufficient to cause apoptosis independent of additional signals. The crystal and solution structures of a Bcl-2 family member, Bcl-xL is like this: The structures consist of two central, primarily hydrophobic α-helices, which are surrounded by amphipathic helices. A 60-residue loop connecting helices αl and α2 was found to be flexible and non-essential for anti-apoptotic activity. Bcl-xL is chareacterized as important factors in autophagy, inhibiting Beclin 1-mediated autophagy by binding to Beclin 1. In addition, Beclin 1, Bcl-2 and Bcl-xL can cooperate with Atg5 or Ca2+ to regulate both autophagy and apoptosis. Bcl-xL is also implicated in anoxia induced cell death. The pathway is initiated by the loss of function of the prosurvival Bcl-2 family members Mcl-1 and Bcl-2 / Bcl-XL, resulting in Bax- or Bak-dependent release of cytochrome c and subsequent caspase-9-dependent cell death. Thus, Bcl-xL, the well-characterized apoptosis guards, appears to be important in cell death. |
| Reference |
