Mouse Carbonic Anhydrase IV / Car4 Protein (His Tag)
AW456718,Ca4,Car4,RP23-167D6.1
- 100ug (NPP2667) Please inquiry
| Catalog Number | P50350-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | AW456718,Ca4,Car4,RP23-167D6.1 |
| Molecular Weight | The secreted recombinant mouse Car4 consists of 271 amino acids and has a calculated molecular mass of 31 kDa as estimated in SDS-PAGE under reducing conditions. |
| predicted N | Glu 18 |
| SDS-PAGE | 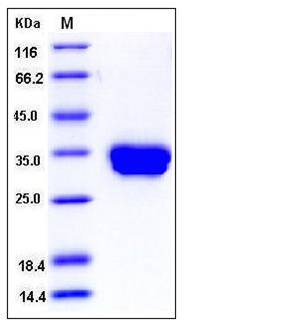 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse Car4 (NP_031633.1) without the C-terminal propeptide (Met 1-Ser 277) was expressed,with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its esterase activity . The specific activity is >10 pmoles/min/μg . |
| Research Area | Cancer |Signal transduction |Metabolism |Mitochondrial |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The carbonic anhydrases (or carbonate dehydratases) are classified as metalloenzyme for its zinc ion prosthetic group and form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons, a reversible reaction that takes part in maintaining acid-base balance in blood and other tissues. The carbonic anhydrasekl (CA) family consists of at least 11 enzymatically active members and a few inactive homologous proteins. Carbonic anhydrase IV (CAIV) is a membrane-associated enzyme anchored to plasma membrane surfaces by a phosphatidylinositol glycan linkage. CAIV is a high-activity isozyme in CO2 hydration comparable to that of CAII. Furthermore, CAIV is more active in HCO3- dehydration than is CAII. However, the esterase activity of CAIV is decreased 150-fold compared to CAII. |
| Reference |
