Mouse Carbonic Anhydrase XII / Car12 Protein (His Tag)
2310047E01Rik,AI314958,Car12
- 100ug (NPP2671) Please inquiry
| Catalog Number | P50014-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | 2310047E01Rik,AI314958,Car12 |
| Molecular Weight | The secreted recombinant mouse CA12 consists of 288 amino acids and has a calculated molecular mass of 32.8 kDa. As a result of glycosylation, the recombinant protein migrates as an approximately 40-45 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Ala 25 |
| SDS-PAGE | 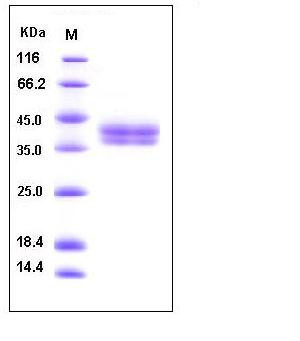 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Ser 301) of mouse CA12 (NP_848483.2) precursor was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its esterase activity. The specific activity is >50 pmoles/min/μg, as measured with 1 mM 4-Nitrophenyl acetate and 0.4 μg enzyme at 400 nm in 100 μL of 12.5 mM Tris, 75 mM NaCl, pH 7.5. |
| Research Area | Cardiovascular |Cardiovascular disease Therapeutic Targets |Hypertension Therapeutic Targets |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Carbonic anhydrases (CAs) are a large family of zinc metalloenzymes first discovered in 1933 that catalyze the reversible hydration of carbon dioxide. CAs participate in a variety of biological processes, including respiration, calcification, acid-base balance, bone resorption, and the formation of aqueous humor, cerebrospinal fluid, saliva, and gastric acid. CA12, also known as Car12 and carbonic anhydrase XII, is a type I membrane enzyme of an N-terminal extracellular catalytic domain, a membrane-spanning α-helix, and a small intracellular C-terminal domain. It is highly expressed in colon, kidney, prostate, intestine and activated lymphocytes and moderately expressed in pancreas, ovary, and testis. Overexpression of the CA12 is observed in certain human cancers and is used as a tumor marker. rmCA12 corresponds to the extracellular domain and has both carbonic anhydrase activity and esterase activity. |
| Reference |
