Mouse Carboxypeptidase M / CPM Protein (His Tag)
1110060I01Rik,5730456K23Rik,AA589379,E030045M14Rik
- 100ug (NPP3274) Please inquiry
| Catalog Number | P50990-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | 1110060I01Rik,5730456K23Rik,AA589379,E030045M14Rik |
| Molecular Weight | The secreted recombinant mouse CPM comprises 416 amino acids and has a calculated molecular mass of 47.8 kDa. The apparent molecular mass of rmCPM is approximately 52 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Leu 18 |
| SDS-PAGE | 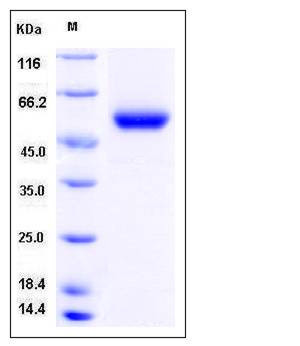 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain of mouse CPM isoform 1 (Q80V42-1) (Met 1-His 422), without the propeptide, was fused with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to release Larginine from BenzoylAlaArg, with detection of the arginine amino group by ophthaldialdehyde. The specific activity is >40,000 pmoles/min/μg. |
| Research Area | Immunology |Signal Transduction |Growth Factor & Receptor |Hormones |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Carboxypeptidase M, also known as CPM, is a membrane-bound arginine/lysine carboxypeptidase which is a member of the carboxypeptidases family. These enzymes remove C-terminal amino acids from peptides and proteins and exert roles in the physiological processes of blood coagulation/fibrinolysis, inflammation, food digestion and pro-hormone and neuropeptide processing. Among the carboxypeptidases CPM is of particular importance because of its constitutive expression in an active form at the surface of specialized cells and tissues in the human body. CPM in the brain appears to be membrane-bound via a phosphatidylinositol glycan anchor. CPM is widely distributed in a variety of tissues and cells. The amino acid sequence of CPM indicated that the C-terminal hydrophobic region might be a signal for membrane attachment via a glycosylphosphatidylinositol (GPI) anchor. CPM is involved in peptide metabolism on both the cell surface and in extracellular fluids. CPM functions not only as a protease but also as a binding partner in cell-surface protein-protein interactions. |
| Reference |
