Mouse Clusterin / Apolipoprotein J / Apo-J / CLU Protein (His Tag)
AI893575,ApoJ,Cli,D14Ucla3,Sgp-2,Sgp2,SP-40,Sugp-2
- 100ug (NPP3264) Please inquiry
| Catalog Number | P50485-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | AI893575,ApoJ,Cli,D14Ucla3,Sgp-2,Sgp2,SP-40,Sugp-2 |
| Molecular Weight | The full length of recombinant mouse CLU comprises 438 amino acids and has a calculated molecular mass of 50.8 kDa. The apparent molecular mass of the recombinant protein is approximately 32, 42 and 65 kDa in SDS-PAGE under reducing conditions, corresponding to the cleaved β chain, α chain and the full length respectively. |
| predicted N | Glu 22 (β chain) & Ser 227(α chain) |
| SDS-PAGE | 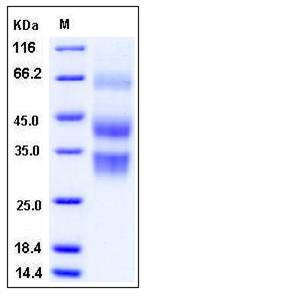 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse CLU (NP_038520.2) (Met 1-Glu 448) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Signaling Pathway |Representative pathway |Apoptosis Signaling pathway |Other Apoptosis Molecules | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Clusterin, also known as complement-associated protein SP-40, Complement cytolysis inhibitor, Apolipoprotein J, Testosterone-repressed prostate message 2, Aging-associated gene 4 protein, CLU and APOJ, is a secreted protein which belongs to the clusterin family. Clusterin/Apolipoprotein J/Apo-J is an enigmatic glycoprotein with a nearly ubiquitous tissue distribution and an apparent involvement in biological processes ranging from mammary gland involution to neurodegeneration in Alzheimer's disease. Its major form, a heterodimer, is secreted and present in physiological fluids, but truncated forms targeted to the nucleus have also been identified. Clusterin/Apolipoprotein J/Apo-J is a widely distributed glycoprotein with a wide range of biologic properties. A prominent and defining feature of clusterin is its marked induction in such disease states as glomerulonephritis, cystic renal disease, renal tubular injury, neurodegenerative conditions, atherosclerosis, and myocardial infarction. Upregulation of clusterin mRNA and protein levels detected in diverse disease states and in in vitro systems have led to suggestions that it functions in membrane lipid recycling, in apoptotic cell death, and as a stress-induced secreted chaperone protein, amongst others. |
| Reference |
