Mouse ENPEP / Aminopeptidase A Protein (His Tag)
6030431M22Rik,APA,Bp-1/6C3,ENPEP,Ly-51,Ly51
- 100ug (NPP2717) Please inquiry
| Catalog Number | P50082-M07B |
|---|---|
| Organism Species | Mouse |
| Host | Baculovirus-Insect Cells |
| Synonyms | 6030431M22Rik,APA,Bp-1/6C3,ENPEP,Ly-51,Ly51 |
| Molecular Weight | The recombinant mouse ENPEP consists of 921 amino acids and predicts a molecular mass of 106 KDa. It migrates as an approximately 113 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 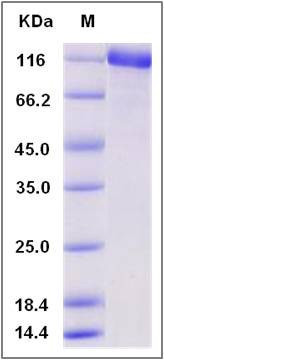 |
| Purity | > 91 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of mouse ENPEP (P16406) (Arg41-Pro945) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, Glu-7-amido-4-methyl coumarin. The specific activity is > 200 pmoles/min/μg. |
| Research Area | Signaling |Signal Transduction |Hormones |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | ENPEP, also known as aminopeptidase A, is a member of the peptidase M1 family. Members of this family are involved in response to cadmium ion and proteolysis. They located in 6 components and are expressed in 26 plant structures. ENPEP is expressed by epithelial cells of the proximal tubule cells and the glomerulus of the nephron. It also can be detected in a variety of other tissues. ENPEP probably plays a role in regulating growth and differentiation of early B-lineage cells. It also may play a role in the catabolic pathway of the renin-angiotensin system. ENPEP is a zinc-dependent membrane-bound aminopeptidase that catalyzes the cleavage of glutamatic and aspartatic amino acid residues from the N-terminus of polypeptides. It degrades vasoconstricting angiotensin II into angiotensin III and therefore helps to regulate blood pressure. |
| Reference |
