Mouse Galectin-1 / LGALS1 Protein
AA410090, Gal-1, Galbp, L-14.5, L14, Lect14, galectin-1
- 100ug (NPP3335) Please inquiry
| Catalog Number | P50100-MNAE |
|---|---|
| Organism Species | Mouse |
| Host | E. coli |
| Synonyms | AA410090, Gal-1, Galbp, L-14.5, L14, Lect14, galectin-1 |
| Molecular Weight | The recombinant mouse Lgals1 consists of 135 amino acids and migrates as an approximately 15 kDa band as predicted in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 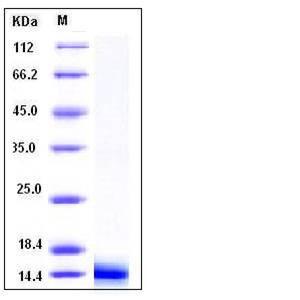 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse Lgals1 (P16045) (Met 1-Glu 135) was expressed and purified. |
| Bio-activity | Measured by its ability to agglutinate human red blood cells. The ED50 for this effect is typically 1-5 μg/ml. |
| Research Area | Cancer |Invasion microenvironment |Adhesion molecule |Cell adhesion |Lectin |Galectin | |
| Formulation | Lyophilized from sterile PBS, 100mM β-Lactose, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Galectin-1 (Gal-1, GAL1), is a member of the galectins, a family of animal lectins ranging from Caenorhabditis elegans to humans, which is defined by their affinity for beta-galactosides and by significant sequence similarity in the carbohydrate-binding site. It is a homodimer with a subunit molecular mass of 14.5 kDa, which contains six cysteine residues per subunit. The cysteine residues should be in a free state in order to maintain a molecular structure that is capable of showing lectin activity. This endogenous lectin widely expressed at sites of inflammation and tumour growth, has been postulated as an attractive immunosuppressive agent to restore immune cell tolerance and homeostasis in autoimmune and inflammatory settings. On the other hand, galectin-1 contributes to different steps of tumour progression including cell adhesion, migration and tumour-immune escape, suggesting that blockade of galectin-1 might result in therapeutic benefits in cancer. Several potential glycoprotein ligands for galectin-1 have been identified, including lysosome-associated membrane glycoproteins and fibronectin, laminin, as well as T-cell glycoproteins CD43 and CD45. Evidence points to Gal-1 and its ligands as one of the master regulators of such immune responses as T-cell homeostasis and survival, T-cell immune disorders, inflammation and allergies as well as host-pathogen interactions. |
| Reference |
