Mouse Legumain/ LGMN Protein (His Tag)
AEP,AI746452,AU022324,Prsc1
- 100ug (NPP3389) Please inquiry
| Catalog Number | P50051-M07H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | AEP,AI746452,AU022324,Prsc1 |
| Molecular Weight | The secreted recombinant mouse LGMN consists of 434 amino acids and has a calculated molecular mass of 49.8 kDa. As a result of glycosylation, the recombinant protein migrates as an approximately 55 kDa protein in SDS-PAGE under reducing conditions. |
| predicted N | Thr 22 |
| SDS-PAGE | 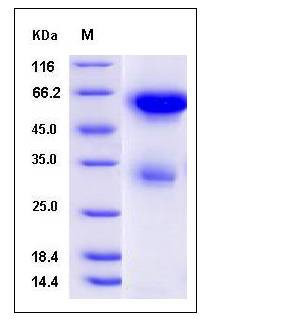 |
| Purity | > 75 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Val 18-Tyr 435) of mouse LGMN (NP_035305.1) precursor was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, N-carbobenzyloxy-Ala-Ala-Asn-7-amido-4-methyl coumarin(Z-AAN-AMC). The specific activity is > 350 pmoles/min/μg. (Activation description: The enzyme achieves its activity under acidic pH) |
| Research Area | Immunology |Signal Transduction |Metabolism |Pathways and Processes |Endocrine metabolism |Hormone biosynthesis | |
| Formulation | Supplied as sterile 25mM Tris, 0.15M NaCl, 20% Glycerol, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | The Mammalian Legumain, also known as LGMN, also called asparaginyl endopeptidase (AEP), is a cysteine protease belonging to peptidase family C13 with a strict specificity for hydrolysis of asparaginyl bonds. Known previously only from plants and invertebrates, Legumain is discovered as a lysosomal endopeptidase in mammals. Mammalian Legumain is a cysteine endopeptidase, inhibited by iodoacetamide and maleimides, but unaffected by compound E64. The Mammalian Legumain is involved in the processing of bacterial peptides and endogenous proteins for MHC class II presentation in the lysosomal/endosomal systems. Legumain has been observed to be highly expressed in several types of solid tumors. It was demonstrated in membrane-associated vesicles concentrated at the invadopodia of tumor cells and on cell surfaces where it colocalized with integrins. Legumain was demonstrated to activate progelatinase A. Cells overexpressing Legumain possessed increased migratory and invasive activity in vitro and adopted an invasive and metastatic phenotype in vivo, inferring significance of Legumain in tumor invasion and metastasis. In addition, Legumain is expressed in both murine and human atherosclerotic lesions. The macrophage-specific expression of Legumain in vivo and ability of Legumain to induce chemotaxis of monocytes and endothelial cells in vitro suggest that Legumain may play a functional role in atherogenesis. |
| Reference |
