Mouse PLA2G1B / Phospholipase-A2 Protein (His Tag)
Pla2a,sPLA2IB
- 100ug (NPP3444) Please inquiry
| Catalog Number | P50329-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | Pla2a,sPLA2IB |
| Molecular Weight | The secreted recombinant mouse PLA2G1B consists of 142 amino acids and has a predicted molecular mass of 16.3 kDa. rmPLA2G1B migrates as an approximately 18 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Ala 16 |
| SDS-PAGE | 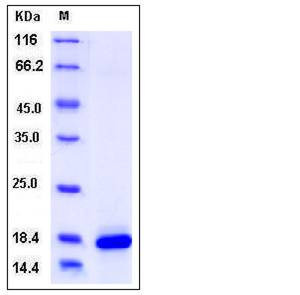 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse PLA2G1B (NP_035237.1) extracellular domain (Met 1-Cys 146) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Signaling Pathway |Lipid Signaling |PLA |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Mouse phospholipase A2, also known as Phosphatidylcholine 2-acylhydrolase 1B, Group IB phospholipase A2, PLA2 and PLA2G1B, is a secreted protein which belongs to the phospholipase A2 family. Phospholipase A2 / PLA2G1B catalyzes the release of fatty acids from glycero-3-phosphocholines. The best known varieties are the digestive enzymes secreted as zymogens by the pancreas of mammals. Sequences of pancreatic Phospholipase A2 / PLA2G1B enzymes from a variety of mammals have been reported. One striking feature of these enzymes is their close homology to venom phospholipases of snakes. Other forms of Phospholipase A2 / PLA2G1B have been isolated from brain, liver, lung, spleen, intestine, macrophages, leukocytes, erythrocytes, inflammatory exudates, chondrocytes, and platelets. Mice lacking in Phospholipase A2 / PLA2G1B are resistant to obesity and diabetes induced by feeding a diabetogenic high-fat/high-carbohydrate diet. Oral supplementation of a diabetogenic diet with the PLA2G1B inhibitor methyl indoxam effectively suppresses diet-induced obesity and diabetes. PLA2G1B inhibition may be a potentially effective oral therapeutic option for treatment of obesity and diabetes. |
| Reference |
