Mouse Podoplanin / PDPN Protein (His & Fc Tag)
Gp38,OTS-8,RANDAM-2,T1-alpha,T1a,T1alpha
- 100ug (NPP3451) Please inquiry
| Catalog Number | P50256-M03H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | Gp38,OTS-8,RANDAM-2,T1-alpha,T1a,T1alpha |
| Molecular Weight | The recombinant mouse PDPN/Fc is a disulfide-linked homodimer after removal of the signal peptide. The reduced monomer consists of 367 amino acids and has a predicted molecular mass of 40.6 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rmPDPN/Fc monomer is approximately 60-65 kDa due to glycosylation. |
| predicted N | Gly 23 |
| SDS-PAGE | 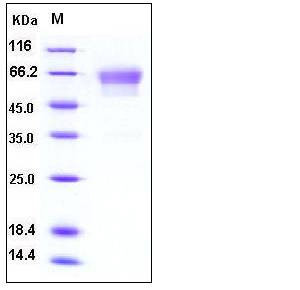 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Leu 141) of mouse PDPN (NP_034459.2) precursor was fused with the C-terminal polyhistidine-tagged Fc region of human IgG1 at the C-terminus. |
| Bio-activity | Immobilized mouse PDPN-Fch at 10 μg/ml (100 μl/well) can bind biotinylated human CLEC1B-His (P10976-H07H), The EC50 of biotinylated human CLEC1B-His (P10976-H07H) is 0.04-0.08 μg/ml. |
| Research Area | Developmental Biology |Organogenesis |Skeletal development |Osteogenesis Markers |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Podoplanin, also known as PDPN, is a type-I integral membrane glycoprotein with diverse distribution in human tissues. The physiological function of this protein may be related to its mucin-type character. The homologous protein in other species has been described as a differentiation antigen and influenza-virus receptor. The specific function of this protein has not been determined. Alternatively spliced transcript variants encoding different isoforms have been identified.PDPN is a mucin-type glycoprotein negatively charged by extensive O-glycosylation and a high content of sialic acid, which expresses the adhesive property. It is selectively expressed in lymphatic endothelium as well as lymphangiomas, Kaposi sarcomas, and in a subset of angiosarcomas with probable lymphatic differentiation. PDPN may contribute to form odontoblastic fiber or function as the anchorage to the tooth development and in proliferating epithelial cells of cervical loop and apical bud. The intensity of podoplanin expression is negatively correlated with the expression of CD34 and factor VIII. Podoplanin would be useful as a diagnostic marker for epithelioid hemangioendothelioma in liver tumors. |
| Reference |
