Mouse Prostasin / PRSS8 Protein (aa 30-289, His Tag)
2410039E18Rik,AI313909,C79772,CAP1,fr,mCAP1
- 100ug (NPP3454) Please inquiry
| Catalog Number | P50120-M08B |
|---|---|
| Organism Species | Mouse |
| Host | Baculovirus-Insect Cells |
| Synonyms | 2410039E18Rik,AI313909,C79772,CAP1,fr,mCAP1 |
| Molecular Weight | The secreted recombinant mouse PRSS8 consists of 270 amino acids and has a calculated molecular mass of 29.3 kDa. It migrates as an approximately 35 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Ala 30 |
| SDS-PAGE | 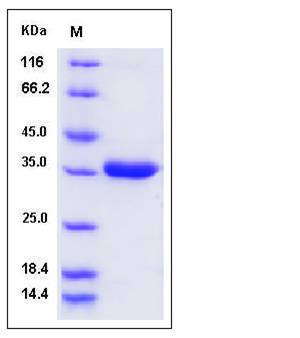 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse PRSS8 (EDL17608.1 ) (Ala 30-Gln 289) was expressed,fused with a polyhistidine tag at the C-terminus and a signal peptide at the N-terminus. |
| Bio-activity | |
| Research Area | Developmental Biology |Embryogenesis |Germ Layer Formation |Ectoderm Marker |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Prostasin (Prss8), also known as channel activating protease 1 (CAP1), is a trypsinlike serine peptidase, and plays important roles in epithelial physiology. It is originally purified as an active, soluble enzyme from human seminal fluid and is highly expressed in prostate, lung, kidney, salivary gland and pancreas. Prostasin is expressed as a glycosyl-phosphatidylinositol (GPI)-anchored membrane protein in prostate epithelial cells, and also exists as a secreted proteolytic enzyme possibly via tryptic cleavage of its COOH-terminal hydrophobic domain. Prostasin is found to activate the epithelial sodium channel (ENaC) which is tightly regulated and is critical for maintaining salt and fluid balance in the lung and kidney in both normal and pathological conditions. Accordingly, prostasin has been proposed as a target for therapeutic inhibition in cystic fibrosis. In addition, prostasin inhibits prostate and breast cancer cell invasion in vitro, suggesting a functional role as a suppressor of tumor invasion, as well as a regulator of gene expression during inflammation. |
| Reference |
