Mouse S100A15 / S100A7A Protein (His & MBP Tag)
AY465109,Gm1020,S100a15,S100a15a,S100a17l1,S100A7f,S100A7L1
- 100ug (NPP2787) Please inquiry
| Catalog Number | P50226-M10E |
|---|---|
| Organism Species | Mouse |
| Host | E. coli |
| Synonyms | AY465109,Gm1020,S100a15,S100a15a,S100a17l1,S100A7f,S100A7L1 |
| Molecular Weight | The recombinant mouse S100A7A/MBP fusion protein consists of 505 amino acids and has a calculated molecular mass of 56.5 kDa. It migrates as an approximately 50 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 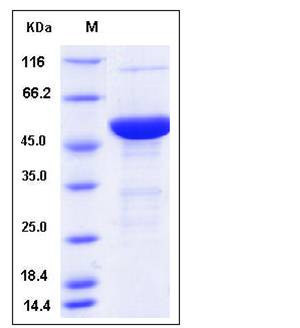 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse S100A7A (Q6S5I3) (Met 1-Tyr 108) was fused with the N-terminal polyhistidine-tagged MBP tag at the N-terminus. |
| Bio-activity | |
| Research Area | Neuroscience |Neurotransmission |Calcium Signaling |Calcium Binding Protein |S100 Protein |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Koebnerisin is also known as protein S100-A7A (S100A7A), S100 calcium-binding protein A7-like 1 (S100A7L1) or S100 calcium-binding protein A15 (S100A15). Human S100A7A / S100A15 is a novel member of the S100 family of EF-hand calcium-binding proteins and was recently identified in psoriasis, where it is significantly upregulated in lesional skin. S100A7 is expressed by both normal cultured and malignant keratinocytes and malignant breast epithelial cells within ductal carcinoma in situ, suggesting an association with abnormal pathways of differentiation. S100A7 plays a role in the pathogenesis of inflammatory skin disease, as a chemotactic factor for hematopoietic cells. It also plays a role in early stages of breast tumor progression in association with the development of the invasive phenotype. The association of the 11.2 kDa S100A7A / S100A15 with psoriasis suggests that it contributes to the pathogenesis of the disease and could provide a molecular target for therapy. |
| Reference |
