Mouse S100A6 Protein
2A9,5B10,Cacy,CALCYCLIN,PRA
- 100ug (NPP3466) Please inquiry
| Catalog Number | P50229-MNAE |
|---|---|
| Organism Species | Mouse |
| Host | E. coli |
| Synonyms | 2A9,5B10,Cacy,CALCYCLIN,PRA |
| Molecular Weight | The recombinant mouse S100A6 consisting of 89 amino acids and has a calculated molecular mass of 10 kDa as estimated in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 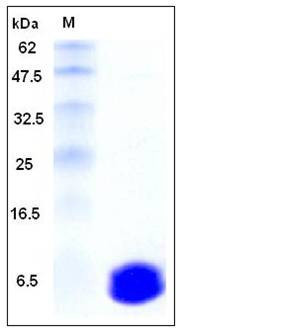 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse S100A6 (NP_035443.1) (Met 1-Lys 89) was expressed. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Neurotransmitter Receptors, Transporters, and Ion Channels |Calcium-binding Proteins and Related Molecules |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | S100 protein is a family of low molecular weight protein found in vertebrates characterized by two EF-hand calcium-binding motifs. There are at least 21 different S100 proteins, and the name is derived from the fact that the protein is 100% soluble in ammonium sulfate at neutral pH. Most S100 proteins are disulfide-linked homodimer, and is normally present in cells derived from the neural crest, chondrocytes, macrophages, dendritic cells, etc. S100 proteins have been implicated in a variety of intracellular and extracellular functions. They are involved in regulation of protein phosphorylation, transcription factors, the dynamics of cytoskeleton constituents, enzyme activities, cell growth and differentiation, and the inflammatory response. S100A6 (S100 calcium binding protein A6) is a member of the S100 family of proteins, and functions in prolactin secretion, and exocytosis. Chromosomal rearrangements and altered expression of S100A6 have been implicated in melanoma. |
| Reference |
