Mouse SIRPB1A / SIRP beta 1 Protein (His Tag)
9930027N05Rik,SIRP-beta,Sirpb,Sirpb1
- 100ug (NPP3494) Please inquiry
| Catalog Number | P50816-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | 9930027N05Rik,SIRP-beta,Sirpb,Sirpb1 |
| Molecular Weight | The secreted recombinant mouse SIRPB1A comprises 348 amino acids and has a calculated molecular mass of 39.1 kDa. As a result of glycosylation, the apparent molecular mass of the recombinant protein is approximately 55-60 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ala 27 |
| SDS-PAGE | 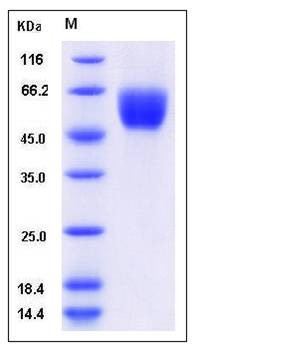 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse SIRPB1A (BAD26610.1) (Met 1-Lys 363) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | SIRPB1A (Signal-regulatory protein beta 1A), also known as SIRP beta 1, belongs to signal-regulatory-protein (SIRP) family, and immunoglobulin superfamily. Signal-regulatory proteins (SIRPs) are cell-surface glycoproteins expressed on myeloid and neural cells that have been shown to recruit SH2 domain-containing protein phosphatase 1 (SHP-1) and SHP-2 and to regulate receptor tyrosine kinase-coupled signaling. SIRP are classified as SIRP alpha molecules, containing a 110- to 113-amino acid long, or SIRP beta molecules, with a 5-amino acid long intracytoplasmic domain. SIRP beta 1 is a new DAP12-associated receptor involved in the activation of myeloid cells, which contains a short cytoplasmic domain that lacks sequence motifs capable of recruiting SHP-1 and SHP-2. SIRP beta 1. SIRP beta 1 acts as an activating isoform of SIRP alpha molecules, confirming the co-existence of inhibitory ITIM-bearing molecules, recruiting SHP-1 and SHP-2 protein tyrosine phosphatases, and activating counterparts, whose engagement couples to protein tyrosine kinases via ITAM-bearing molecules. |
| Reference |
