Mouse Semaphorin 3A / SEMA3A Protein (Fc Tag)
coll-1,Hsema-I,SEMA1,Semad,SemD
- 100ug (NPP2789) Please inquiry
| Catalog Number | P50631-M01H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | coll-1,Hsema-I,SEMA1,Semad,SemD |
| Molecular Weight | The recombinant mouse SEMA3A/Fc is a disulfide-linked homodimer The reduced monomer consists of 781 amino acids and has a predicted molecular mass of 87.7 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rm SEMA3A/Fc monomer is approximately 100 kDa due to glycosylation. |
| predicted N | Glu |
| SDS-PAGE | 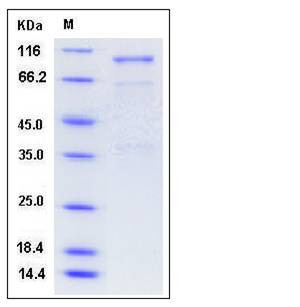 |
| Purity | > 80 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the N-terminal fragment (Lys 26-Phe 546) of mouse SEMA3A (O08665) was fused with the Fc region of human IgG1 at the N-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Heart |Cardiac arrhythmias |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Semaphorins are a family of secreted and cell-bound signaling molecules defined by the presence of a common 500 aa Sema domain. They are best characterized in relation to axon guidance during development of the nervous system. The functions of Semaphorins 3A (SEMA3A) are mediated primarily through binding to the Neuropilin-1 (Npn-1) and Plexin-A1 coreceptor complex. Neuropilins lack a signaling-competent cytoplasetmic domain and ensure semaphorin binding, whereas the transmembrane receptor plexin mediates the intracellular response. As the first identified vertebrate semaphorin, SEMA3A functions either as a chemorepulsive agent inhibiting axonal outgrowth, or as a chemoattractive agent stimulating the growth of apical dendrites. In both cases, the protein is vital for normal neuronal pattern development. Its overexpression is associated with schizophrenia which is seen in various human tumor cell lines, and aberrant release is associated with the progression of Alzheimer's disease |
| Reference |
