Mouse SerpinA10 / ZPI Protein (His Tag)
PZI,ZPI
- 100ug (NPP3474) Please inquiry
| Catalog Number | P50315-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | PZI,ZPI |
| Molecular Weight | The secreted recombinant mouse SERPINA10 comprises 438 amino acids with a predicted molecular mass of 51 kDa. As a result of glycosylation, the apparent molecular mass of the protein is approximately 60-80 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Phe 22 |
| SDS-PAGE | 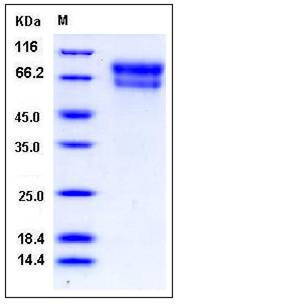 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of mouse SERPINA10 isoform 1 (Q8R121-1) (Met 1-Leu 448) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Blood |Coagulation |Coagulation Cascade |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Mouse protein Z-dependent protease inhibitor, also known as PZ-dependent protease inhibitor, SERPINA10 and ZPI, is a secreted protein which belongs to the serpin family. It is expressed by the liver and secreted in plasma. SERPINA10 / Serpin-A10 inhibits factor Xa activity in the presence of protein Z, calcium and phospholipid. Serpins are a group of proteins with similar structures that were first identified as a set of proteins able to inhibit proteases. The acronym serpin was originally coined because many serpins inhibit chymotrypsin-like serine proteases (serine protease inhibitors).Over 1000 serpins have now been identified, these include 36 human proteins, as well as molecules in plants, fungi, bacteria, archaea and certain viruses. Serpins are the largest and most diverse family of protease inhibitors. Most serpins control proteolytic cascades, certain serpins do not inhibit enzymes, but instead perform diverse functions such as storage (ovalbumin, in egg white), hormone carriage proteins (thyroxine-binding globulin, cortisol-binding globulin) and tumor suppressor genes (maspin). Most inhibitory serpins target chymotrypsin-like serine proteases. These enzymes are defined by the presence of a nucleophilic serine residue in their catalytic site. Some serpins inhibit other classes of protease. A number of such serpins have been shown to target cysteine proteases. These enzymes differ from serine proteases in that they are defined by the presence of a nucleophilic cysteine residue, rather than a serine residue, in their catalytic site. |
| Reference |
