Mouse Serpinb3c Protein (His Tag)
1110001H02Rik,1110013A16Rik,Scca2,Serpinb3c,Serpinb4
- 100ug (NPP3478) Please inquiry
| Catalog Number | P50625-M08B |
|---|---|
| Organism Species | Mouse |
| Host | Baculovirus-Insect Cells |
| Synonyms | 1110001H02Rik,1110013A16Rik,Scca2,Serpinb3c,Serpinb4 |
| Molecular Weight | The recombinant mouse SERPINB3C consists of 397 amino acids and has a calculated molecular mass of 46.5 kDa. The apparent molecular mass of the protein is approximately 46 & 95 kDa in SDS-PAGE under reducing conditions, corresponding to the monomer and dimer. |
| predicted N | Met 1 |
| SDS-PAGE | 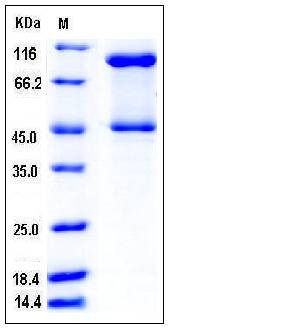 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse SERPINB3C (NP_958751.2) (Met 1-Pro 386) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Fibrinolysis System |
| Formulation | Lyophilized from sterile 50mM Tris 100mM NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Serpins are the largest and most diverse family of serine protease inhibitors which are involved in a number of fundamental biological processes such as blood coagulation, complement activation, fibrinolysis, angiogenesis, inflammation and tumor suppression and are expressed in a cell-specific manner. Serpins are a group of proteins with similar structures that were first identified as a set of proteins able to inhibit proteases. The acronym serpin was originally coined because many serpins inhibit chymotrypsin-like serine proteases (serine protease inhibitors). Over 1000 serpins have been identified. Mouse SerpinB3, also known as Squamous cell carcinoma antigen 1, SCCA-1, SERPINB3, SCCA and SCCA1, is a cytoplasm protein which belongs to the serpin family and Ov-serpin subfamily. SerpinB3 may act as a protease inhibitor to modulate the host immune response against tumor cells. Mouse SerpinB3a and SerpinB3b, but not Serpinb3c, are functional, inhibiting both serine and cysteine proteinases with different inhibitory profiles due to the difference of two amino acids in their reactive site loops. SerpinB3a is ubiquitously expressed in most tissues, whereas expression of SerpinB3b is limited to keratinocytes. SerpinB3a and SerpinB3b may play different roles by inhibiting intrinsic or extrinsic proteinases with different expression distributions and different inhibitory profiles. |
| Reference |
