Mouse Siglec-2 / CD22 Protein (His Tag)
A530093D23,Lyb-8,Lyb8
- 100ug (NPP3490) Please inquiry
| Catalog Number | P51177-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | A530093D23,Lyb-8,Lyb8 |
| Molecular Weight | The recombinant mouse CD22 comprises 701 amino acids and has a predicted molecular mass of 78.8 kDa. The apparent molecular mass of the protein is approximately 116 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Arg 19 |
| SDS-PAGE | 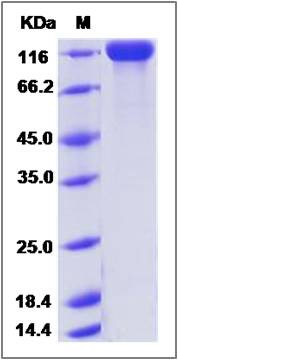 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse CD22 (BAE33829.1)(Met1-Arg708) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |ITIM/ITAM Immunoreceptors and Related Molecules |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | CD22 is a member of the immunoglobulin superfamily, SIGLEC family of lectins. It is first expressed in the cytoplasm of pro-B and pre-B cells, and on the surface as B cells mature to become IgD+. CD22 serves as an adhesion receptor for sialic acid-bearing ligands expressed on erythrocytes and all leukocyte classes. In addition to its potential role as a mediator of intercellular interactions, signal transduction through CD22 can activate B cells and modulate antigen receptor signaling in vitro. The phenotype of CD22-deficient mice suggests that CD22 is primarily involved in the generation of mature B cells within the bone marrow, blood, and marginal zones of lymphoid tissues. CD22 recruits the tyrosine phosphatase Src homology 2 domain-containing phosphatase 1 (SHP-1) to immunoreceptor tyrosine-based inhibitory motifs (ITIMs) and inhibits B-cell receptor (BCR)-induced Ca2+ signaling on normal B cells. CD22 interacts specifically with ligands carrying alpha2-6-linked sialic acids. As an inhibitory coreceptor of the B-cell receptor (BCR), CD22 plays a critical role in establishing signalling thresholds for B-cell activation. Like other coreceptors, the ability of CD22 to modulate B-cell signalling is critically dependent upon its proximity to the BCR, and this in turn is governed by the binding of its extracellular domain to alpha2,6-linked sialic acid ligands. However, genetic studies in mice reveal that some CD22 functions are regulated by ligand binding, whereas other functions are ligand-independent and may only require expression of an intact CD22 cytoplasmic domain at the B-cell surface. CD19 regulates CD22 phosphorylation by augmenting Lyn kinase activity, while CD22 inhibits CD19 phosphorylation via SHP-1. |
| Reference |
