Mouse Trypsin 2 / PRSS2 Protein (His Tag)
Prss2,Ta,Tesp4,Try2,TRY8,TRYP
- 100ug (NPP2803) Please inquiry
| Catalog Number | P50383-M08H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | Prss2,Ta,Tesp4,Try2,TRY8,TRYP |
| Molecular Weight | The recombinant pro form of mouse PRSS2 comprises 242 amino acids and has a predicted molecular mass of 26.2 kDa. The apparent molecular mass of rm PRSS2 is approximately 32 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Phe 16 |
| SDS-PAGE | 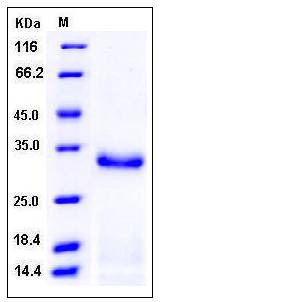 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mouse PRSS2 (NP_033456.1) precursor (Met 1-Asn 246) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, Mca-RPKPVE-Nval-WRK(Dnp)-NH2 (Anaspec, Catalog #27096) . The specific activity is >1500 pmoles/min/μg . (Activation description: The proenzyme needs to be activated by enteropeptidase for an activated form) |
| Research Area | Developmental Biology |Metabolism |Amino Acids |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Mouse Trypsin-2, also known as Trypsin II, Anionic trypsinogen, Serine protease 2, PRSS2 and TRY2, is a secreted protein which belongs to the trypsin serine protease family including Trypsin, PRSS1, PRSS2 and PRSS3. It consists of a signal peptide (residues 1-15), a pro region (residues 16-23), and a proteolytically active mature chain (residues 24-247). PRSS2 contains one peptidase S1 domain. It is secreted into the duodenum, hydrolysing peptides into their smaller building blocks, which is necessary for the uptake of protein in the food. It is secreted by the pancreas in the form of inactive zymogen, trypsinogen and cleaved to its active form in the small intestine when the pancreas is stimulated by cholecystokinin through the common activation mechanism. |
| Reference |
