Mouse VSIG4 Protein (His & Fc Tag)
A530061A11,BC025105,CRIg,Z39IG
- 100ug (NPP3541) Please inquiry
| Catalog Number | P50187-M03H |
|---|---|
| Organism Species | Mouse |
| Host | Human Cells |
| Synonyms | A530061A11,BC025105,CRIg,Z39IG |
| Molecular Weight | The recombinant mouse VSIG4/Fc is a disulfide-linked homodimer after removal of the signal peptide. The reduced monomer consists of 416 amino acids and has a predicted molecular mass of 47 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rmVSIG4/Fc monomer is approximately 60 kDa due to glycosylation. |
| predicted N | His 20 |
| SDS-PAGE | 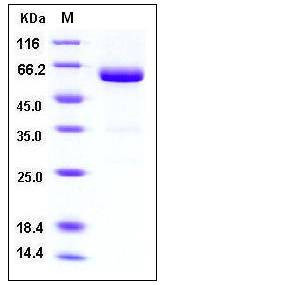 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Pro 187) of mouse VSIG4 (NP_808457.1) precursor was fused with the C-terminal polyhistidine-tagged Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Inflammatory Mediators |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | VSIG4 (V-set and immunoglobulin domain containing 4), also known as complement receptor of the immunoglobulin superfamily (CRIg) and Z39Ig, is a type I transmembrane glycoprotein. It is a B7 family-related protein and an Ig superfamily member. In contrast to the B7 family members which contain two IgG domains, VSIG4 contains one complete V-type I g domain and a truncated C-type I g domain. VSIG4 is exclusively expressed on tissue resident macrophages and binds to multimers of C3b and iC3b that are covalently attached to particle surfaces. No VSIG4 expression appears to be present in T and B cells. VSIG4 functions as a negative regulator of T cell activation, and may be involved in the maintenance of peripheral T cell tolerance, and is also identified as a potent suppressor of established inflammation. Mouse VSIG4 is synthesized as a 280 amino acid precursor that contains a signal sequence, an V-type I g domain (aa 36-115), one potential N-linked glycosylation site, and a single transmembrane domain. The V-type I g domain of mouse VSIG4 shares 86% and 80% aa sequence identity with the V-type I g domains of rat and human VSIG4, respectively. |
| Reference |
