Rat Angiotensinogen / SerpinA8 / AGT Protein (His Tag)
Serpina8
- 100ug (NPP3103) Please inquiry
| Catalog Number | P81380-R08H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | Serpina8 |
| Molecular Weight | The recombinant rat Agt consists 464 amino acids and predicts a molecular mass of 51 kDa. |
| predicted N | Asp 25 |
| SDS-PAGE | 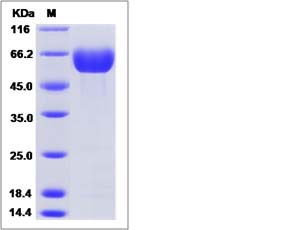 |
| Purity | > 95 % as determined by SDS-PAGE. |
| Protein Construction | A DNA sequence encoding the rat Agt (NP_602308.1) (Met1-Val477) was expressed with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Fibrinolysis System |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Angiotensinogen, also known as AGT and SerpinA8, is a member of the serpin family. It is an α-2-globulin that is produced constitutively and released into the circulation mainly by the liver. Angiotensinogen is a essential component of the renin-angiotensin system (RAS) and a potent regulator of blood pressure. Angiotensinogen can be schematically considered to consist of a combination of an angiotensin I (Ang I) function, located at the N-terminal end, and the presence of a serpin (serine protease inhibitor) structure at the opposite end. Angiotensinogen is cleaved into three chains: Angiotensin-1 (Ang I), Angiotensin-2 (Ang II), and Angiotensin-3 (Ang III). Angiotensin-1 is a substrate of ACE (angiotensin converting enzyme) that removes a dipeptide to yield the physiologically active peptide angiotensin-2. Angiotensin-1 and angiotensin-2 can be further processed to generate angiotensin-3, angiotensin-4. Angiotensin 1-7 is cleaved from angiotensin-2 by ACE2. Angiotensin-2 acts directly on vascular smooth muscle as a potent vasoconstrictor, affects cardiac contractility and heart rate through its action on the sympathetic nervous system. Defects in AGT are associated with susceptibility to essential hypertension and renal tubular dysgenesis (RTD). Several serpins (antithrombin, maspin, pigment epithelial-derived factor, and kallistatin) have been recently shown to exert an antiangiogenic activity, suggesting a common mechanism of endothelial cell proliferation and migration. Angiotensinogen/AGT and its renin-cleaved product, des(Ang I)AGT, are also angiogenesis inhibitors, both in vitro and in vivo at concentrations within the range of those observed in plasma. The Angiotensinogen products, that is angiotensin II and possibly angiotensin II-related products, have been found to act locally in modulating adipose tissue growth in an autocrine/paracrine manner. The transient or chronic overexpression of angiotensinogen in adipose tissue favors lipogenesis in adipocytes and leads to a 'vicious' circle whereby adipose tissue development is further increased. |
| Reference |
