Rat C1 inhibitor / SerpinG1 Protein (His Tag)
Serping1
- 100ug (NPP3106) Please inquiry
| Catalog Number | P81647-R08H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | Serping1 |
| Molecular Weight | The recombinant rat Serping1 consists of 493 amino acids and predicts a molecular mass of 54.7 kDa. |
| predicted N | Asp 23 |
| SDS-PAGE | 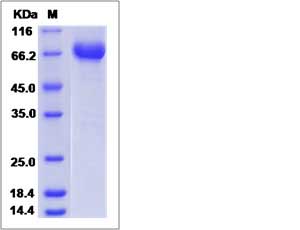 |
| Purity | > 95 % as determined by SDS-PAGE. |
| Protein Construction | A DNA sequence encoding the rat Serping1 (NP_954524.1) (Met1-Ala504) was expressed with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Coagulation |Regulatory |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Plasma protease C1 inhibitor, also known as C1-inhibiting factor, C1-INH, C1 esterase inhibitor, SERPING1 and C1IN, is a serine proteinase inhibitor (serpin) that regulates activation of both the complement and contact systems. By its C-terminal part (serpin domain), characterized by three beta-sheets and an exposed mobile reactive loop, C1-INH binds, and blocks the activity of its target proteases. The N-terminal end (nonserpin domain) confers to C1-INH the capacity to bind lipopolysaccharides and E-selectin. Owing to this moiety, C1-INH intervenes in regulation of the inflammatory reaction. The heterozygous deficiency of C1-INH results in hereditary angioedema (HAE). Owing to its ability to modulate the contact and complement systems and the convincing safety profile, plasma-derived C1 inhibitor is an attractive therapeutic protein to treat inflammatory diseases other than HAE. Deficiency of C1 inhibitor results in hereditary angioedema, which is characterized by recurrent episodes of localized angioedema of the skin, gastrointestinal mucosa or upper respiratory mucosa. C1 inhibitor may prove useful in a variety of other diseases including septic shock, reperfusion injury, hyperacute transplant rejection, traumatic and hemorrhagic shock, and the increased vascular permeability associated with thermal injury, interleukin-2 therapy and cardiopulmonary bypass. |
| Reference |
