Rat CLEC14A / EGFR-5 Protein (Fc Tag)
CLEC14A
- 100ug (NPP3018) Please inquiry
| Catalog Number | P80211-R02H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | CLEC14A |
| Molecular Weight | The recombinant rat CLEC14A/Fc is a disulfide-linked homodimer. The reduced monomer comprises 618 amino acids and has a predicted molecular mass of 67.6 kDa. The apparent molecular mass of the protein is approximately 110 and 37 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Glu 22 |
| SDS-PAGE | 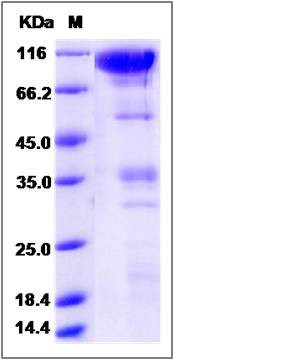 |
| Purity | (75.4+12.7) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the rat CLEC14A (Met1-Thr398) was expressed with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Cytoskeleton / ECM |Cell Adhesion |Lectin |C-tyep lectin | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | C-type lectin domain family 14 member A, also known as Epidermal growth factor receptor 5 and CLEC14A, is a member of the C-type lectin domain (CTLD) family that contains one c-type lectin domain and one EGF-like domain. Mouse CLEC14A is a 459 amino acid single-pass type I membrane protein. The superfamily of proteins containing C-type lectin-like domains (CTLDs) is a large group of extracellular Metazoan proteins with diverse functions. The CTLD structure has a characteristic double-loop ('loop-in-a-loop') stabilized by two highly conserved disulfide bridges located at the bases of the loops, as well as a set of conserved hydrophobic and polar interactions. Members of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily share a common fold and are involved in a variety of functions, such as generalized defense mechanisms against foreign agents, discrimination between healthy and pathogen-infected cells, and endocytosis and blood coagulation. Genome-level studies on human, elegans and melanogaster demonstrated almost complete divergence among invertebrate and mammalian families of CTLD-containing proteins (CTLDcps). The vertebrate CTLDcp families were essentially formed early in vertebrate evolution and are completely different from the invertebrate families. The composition of the CTLDcp superfamily in fish and mammals suggests that large scale duplication events played an important role in the evolution of vertebrates. |
| Reference |
