Rat Cadherin-15 / CDH15 Protein (Fc Tag)
CDH15
- 100ug (NPP3015) Please inquiry
| Catalog Number | P80281-R02H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | CDH15 |
| Molecular Weight | The recombinant rat CDH15 /Fc comprises 822 amino acids and has a predicted molecular mass of 90.6 kDa. The apparent molecular mass of the protein is approximately 118 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Val 22 |
| SDS-PAGE | 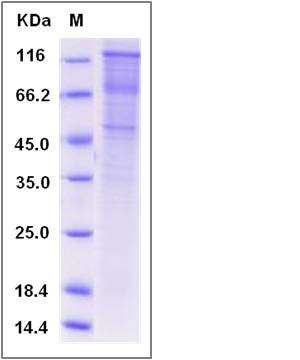 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the rat CDH15 (Q75NI5) (Met1-Gly602) was expressed, fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Cell Biology |Cell Cycle |Cell Differentiation |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cadherin-15, also known as CDH15, is a member of the cadherin superfamily. Cadherins consist of an extracellular domain containing 5 cadherin domains, a transmembrane region, and a conserved cytoplasmic domain. Cadherins are calcium dependent cell adhesion proteins. They preferentially interact with themselves in a homophilic manner in connecting cells; cadherins may thus contribute to the sorting of heterogeneous cell types. Cadherin-15 contains 5 cadherin domains. It is expressed in some normal epithelial tissues and in some carcinoma cell lines. Defects in CDH3 are the cause of ectodermal dysplasia with ectrodactyly and macular dystrophy (EEM), also known as EEM syndrome, Albrectsen-Svendsen syndrome or Ohdo-Hirayama-Terawaki syndrome. Ectodermal dysplasia defines a heterogeneous group of disorders due to abnormal development of two or more ectodermal structures. EEM is an autosomal recessive condition characterized by features of ectodermal dysplasia such as sparse eyebrows and scalp hair, and selective tooth agenesis associated with macular dystrophy and ectrodactyly. |
| Reference |
