Rat Carboxypeptidase B2 / CPB2 Protein (His Tag)
CPB2
- 100ug (NPP3107) Please inquiry
| Catalog Number | P80947-R08H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | CPB2 |
| Molecular Weight | The recombinant rat Cpb2 consists of 412 amino acids and predicts a molecular mass of 48 kDa. |
| predicted N | Phe 22 |
| SDS-PAGE | 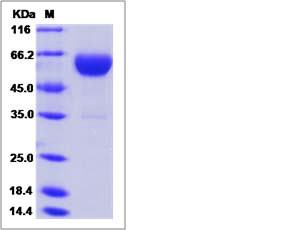 |
| Purity | > 95 % as determined by SDS-PAGE. |
| Protein Construction | A DNA sequence encoding the rat Cpb2 (NP_446069.1) (Met1-Ser422) was expressed with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Complement and Coagulation |Coagulation |Coagulation Cascade |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Carboxypeptidase B2, also known as Carboxypeptidase U, Thrombin-activable fibrinolysis inhibitor, Plasma carboxypeptidase B, CPB2, is a secreted protein which belongs to the peptidase M14 family. Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). CPB2 is activated by thrombin and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates fibrinolysis. CPB2 is synthesized by the liver and circulates in the plasma as a plasminogen-bound zymogen. When it is activated by proteolysis at residue Arg92 by the thrombin / thrombomodulin complex. CPB2 cleaves C-terminal arginine or lysine residues from biologically active peptides such as kinins or anaphylatoxins in the circulation thereby regulating their activities. CPB2 exhibits carboxypeptidase activity and activated CPB2 reduces fibrinolysis by removing the fibrin C-terminal residues that are important for the binding and activation of plasminogen. |
| Reference |
