Rat LAMP1 / CD107a Protein (Fc Tag)
LAMP1
- 100ug (NPP3078) Please inquiry
| Catalog Number | P80226-R02H |
|---|---|
| Organism Species | Rat |
| Host | Human Cells |
| Synonyms | LAMP1 |
| Molecular Weight | The recombinant Rat LAMP1/Fc is a disulfide-linked homodimer. The reduced monomer comprises 591 amino acids and has a predicted molecular mass of 65.1 kDa. The apparent molecular mass of the protein is approximately 123 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ala 22 |
| SDS-PAGE | 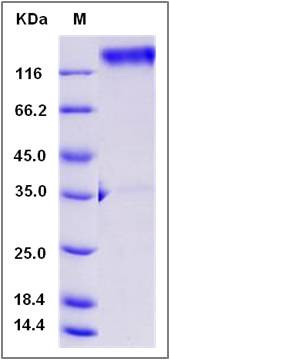 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the Rat LAMP1 (P14562) (Met1-Asn371) was expressed, fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Lysosomal Enzymes |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Lysosome-associated membrane glycoprotein 1, also known as CD107 antigen-like family member A, CD107a, and LAMP1, is a single-pass type I membrane protein which belongs to the LAMP family. CD107a is expressed largely in the endosome-lysosome membranes of cells, but is also found on the plasma membrane (1-2% of total LAMP1). LAMP1 has been implicated in a variety of cellular functions, including cancer metastasis. It has been proposed LAMP1 serves as a therapeutic agent for some cancers, as well as a marker for lysosomal storage disorders and different cell types such as cytotoxic T cells. LAMP2, also known as CD107b, may also play a role in tumor cell metastasis and functions in the protection, maintenance, and adhesion of the lysosome. Cell surface LAMP1 and LAMP2 have been shown to promote adhesion of human peripheral blood mononuclear cells (PBMC) to vascular endothelium, therefore they are possibly involved in the adhesion of PBMCs to the site of inflammation. LAMP-1 is a glycoprotein highly expressed in lysosomal membranes. The present study was initiated to test LAMP-1 mRNA and protein levels in post mortem frontal cortex (area 8) of Alzheimer's disease (AD) stages I-IIA/B and stages V-VIC of Braak and Braak, compared with age-matched controls. LAMP-1 occurred in microglia and multinucleated giant cells in one AD case in whom amyloid burden was cleared following betaA-peptide immunization. In addition, LAMP-1 has been suggested to be a cell surface receptor for a specific amelogenin isoform, leucine-rich amelogenin peptide or LRAP. LAMP-1 can serve as a cell surface binding site for amelogenin on dental follicle cells and cementoblasts. |
| Reference |
