Rat MDHA / MDH1 Protein (His Tag)
MDL1, Mdhl, Mor2
- 100ug (NPP3087) Please inquiry
| Catalog Number | P80232-R08E |
|---|---|
| Organism Species | Rat |
| Host | E. coli |
| Synonyms | MDL1, Mdhl, Mor2 |
| Molecular Weight | The recombinant rat MDH1 comprises 345 amino acids and predicts a molecular mass of 38 kDa. The apparent molecular mass of the rat MDH1 is approximately 39 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 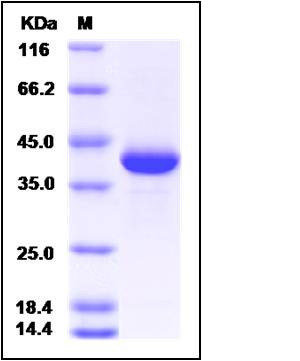 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the rat MDH1 (O88989) (Met 4-Ala 334) was expressed with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Metabolism |Types of disease |Metabolism in Cancer |
| Formulation | Lyophilized from sterile 20mM Tris, 10% glycerol, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Malate dehydrogenases 1(MDH1 / MDHA) is soluable form of malate dehydrogenases. Malate dehydrogenases (MDH) is a group of multimeric enzymes consisting of identical subunits usually organized as either dimer or tetramers with subunit molecular weights of 30-35 kDa. MDH has been isolated from different sources including archaea, eubacteria, fungi, plant and mammals. MDH catalyzes the NAD/NADH-dependent interconversion of the substrates malate and oxaloacetate. This reaction plays a key part in the malate / aspartate shuttle across the mitochondrial membrane, and in the tricarboxylic acid cycle within the mitochondrial matrix. The enzymes share a common catalytic mechanism and their kinetic properties are similar, which demonstrates a high degree of structural similarity. The three-dimensional structures and elements essential for catalysis are conserved between mitochondrial and cytoplasmic forms of MDH in eukaryotic cells even though these isoenzymes are only marginally related at the level of primary structure. |
| Reference |
